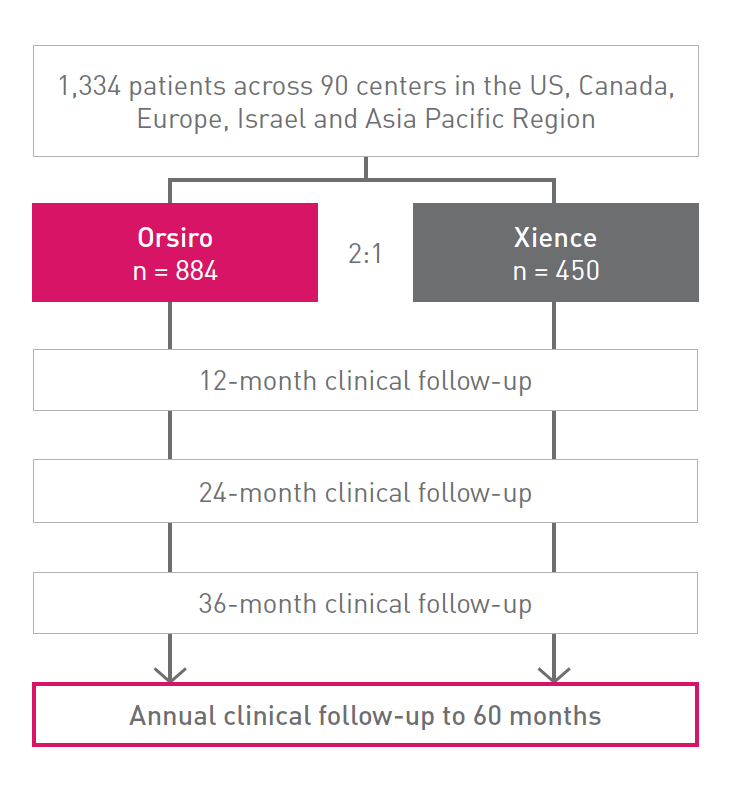
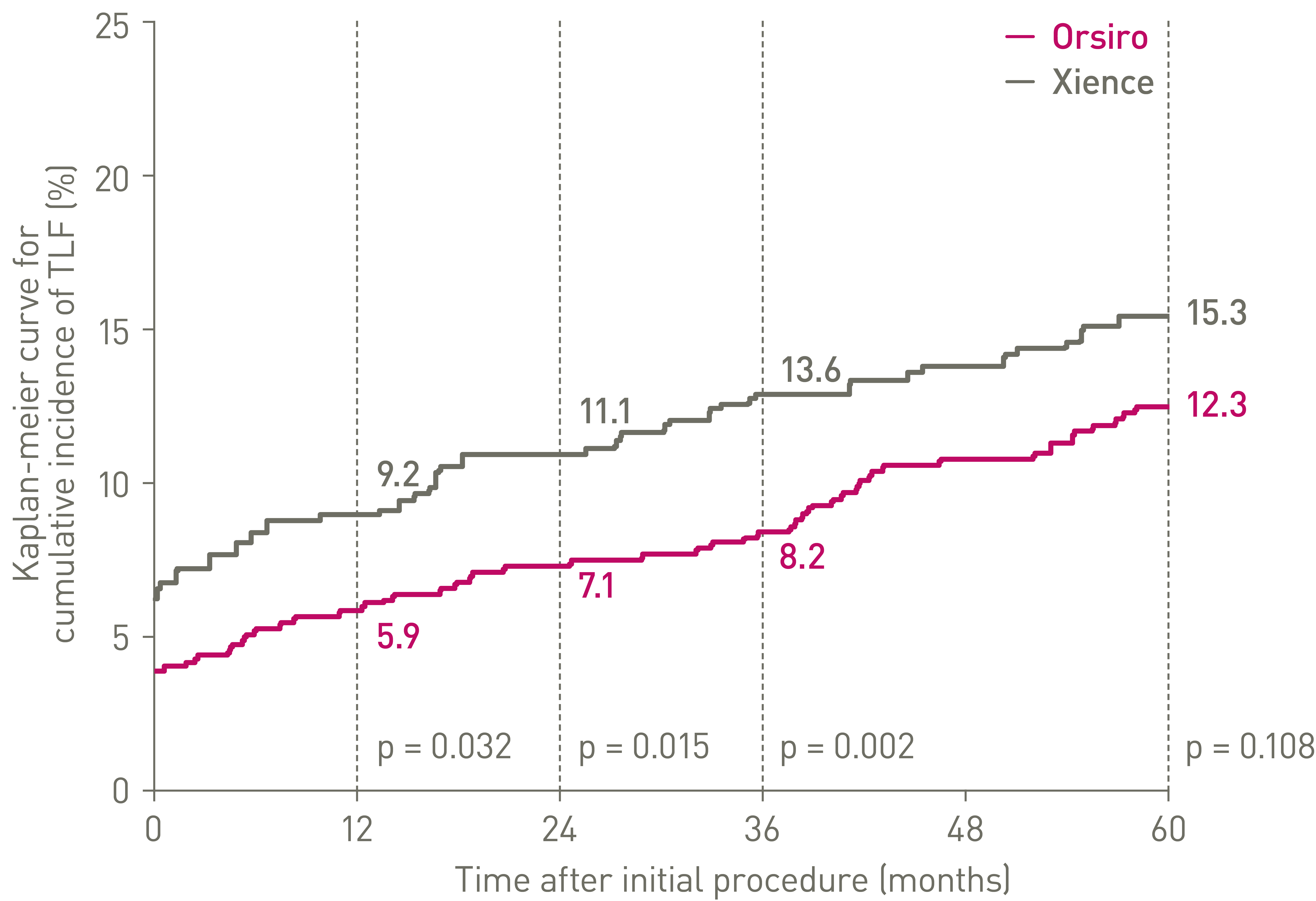
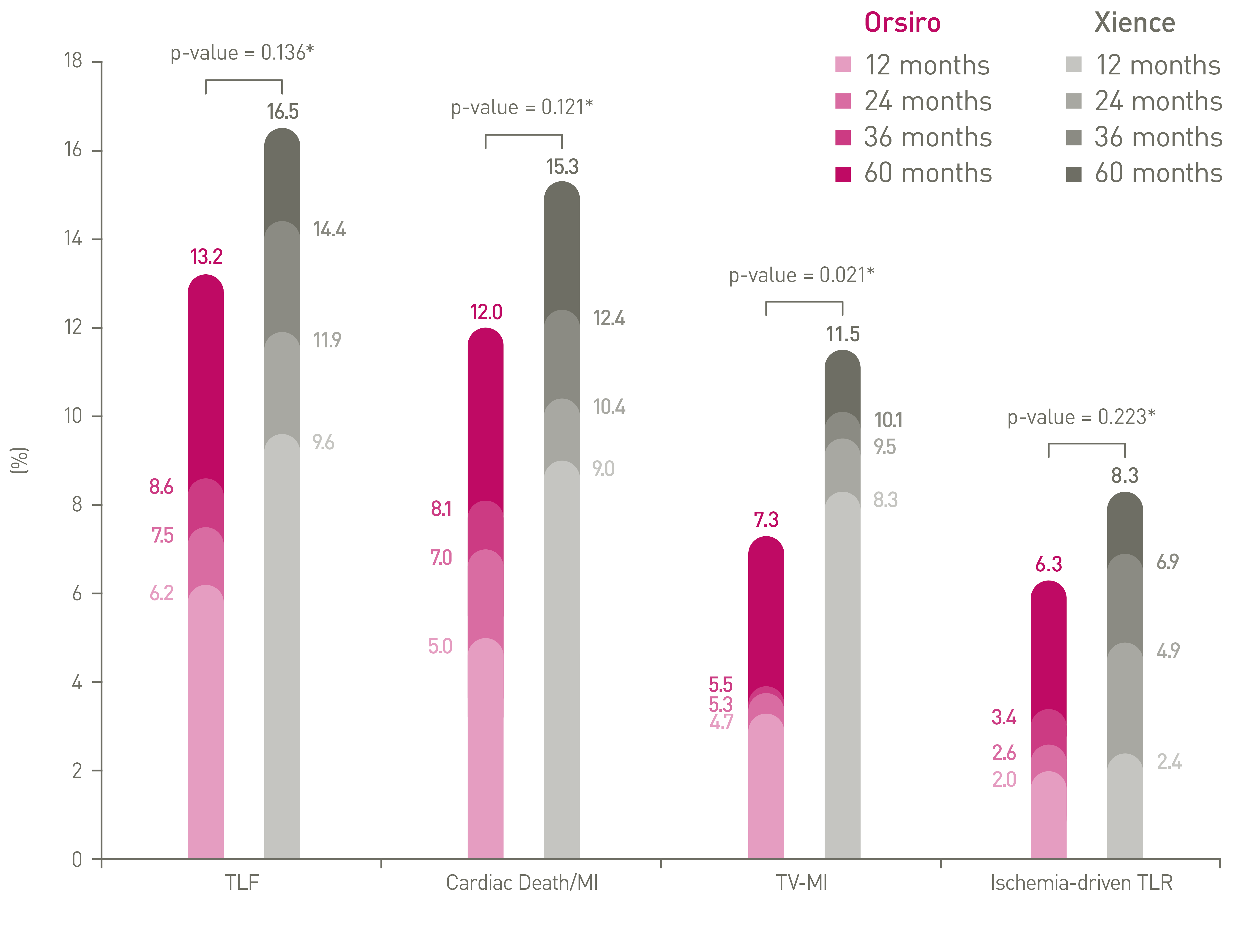
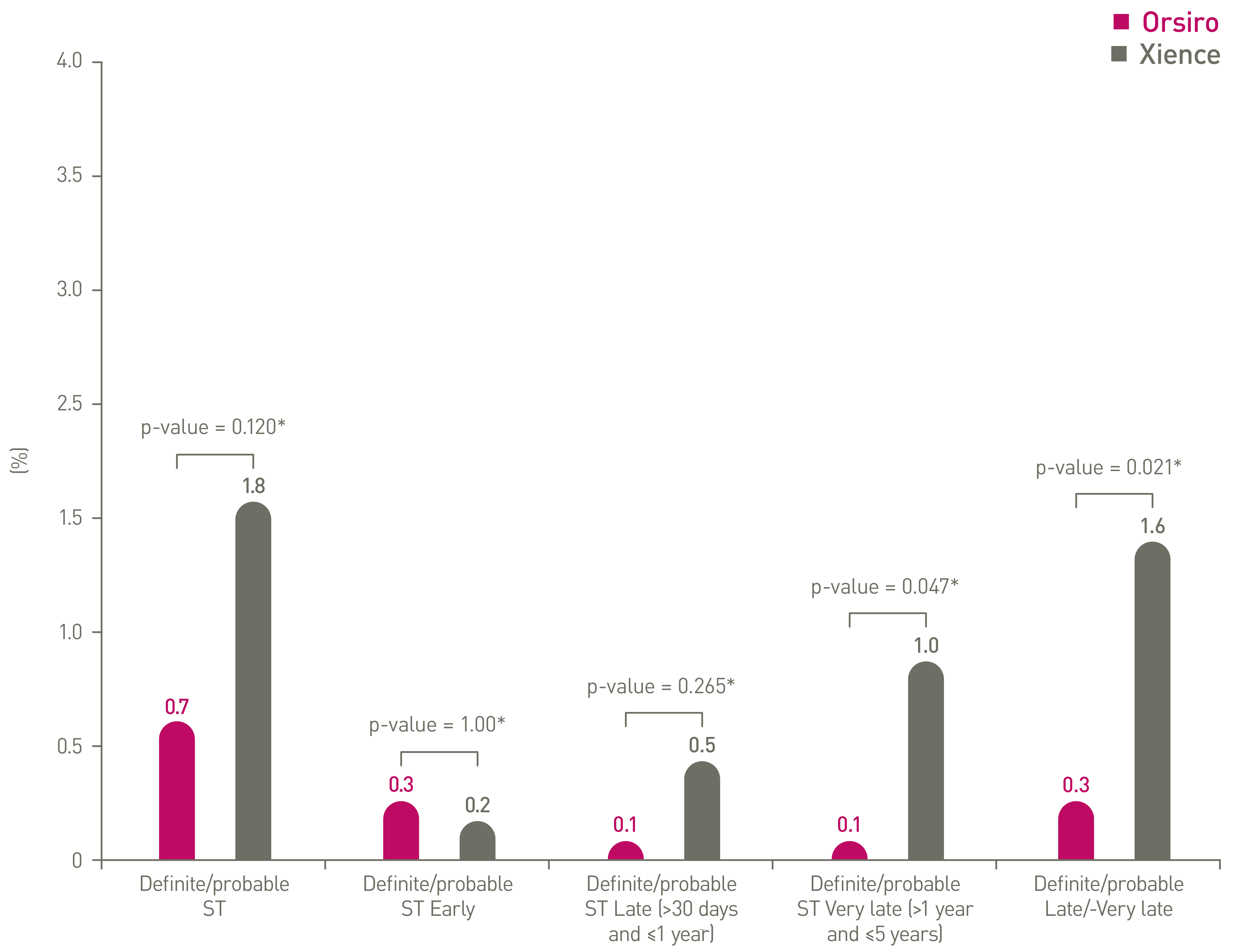
PCI: Percutaneous Coronary Intervention, CABG: Coronary Artery Bypass Graft, DES: Drug Eluting Stent, PTCA: Percutaneous Transluminal Coronary Angioplasty, IDE: Investigational Device Exemption, DP-EES: Durable Polymer Everolimus-Eluting Stents.
*p-values for 60-month frequentist analysis
1. Kandzari D et al. Ultrathin Bioresorbable Polymer Sirolimus-Eluting Stents versus Thin Durable Polymer Everolimus-Eluting Stents for Coronary Revascularization: Final 5-year Outcomes from the Randomized BIOFLOW V Trial, JACC, 2022: NCT02389946; 2. Kandzari D et al. J Am Coll Cardiol. Cardiovasc Interven. 2020, doi: 10.1016/j. jcin.2020.02.019; 3. Kandzari D et al. Ultrathin bioresorbable polymer sirolimus-eluting stents versus thin durable polymer everolimus-eluting stents. Journal of the American College of Cardiology. 2018 Dec 17;72(25):3287-97; 4. Kandzari D, et al. BIOFLOW-V: A Prospective Randomized Multicenter Study to Assess the Safety and Effectiveness of the Orsiro Sirolimus Eluting Coronary Stent System in the Treatment Of Subjects With up to Three De Novo or Restenotic Coronary Artery Lesions Science. Presentation at ESC 2017; 5. Kandzari D et al. Ultrathin, bioresorbable polymer sirolimus-eluting stents versus thin, durable polymer everolimus-eluting stents in patients undergoing coronary revascularisation (BIOFLOW V): a randomised trial. Lancet. 2017 Oct 21; 390(10105):1843-1852.
Clinical data conducted with Orsiro, Orsiro Mission’s predecessor device can be used to illustrate Orsiro Mission clinical outcomes.
Orsiro and Orsiro Mission are a trademarks or registered trademarks of the BIOTRONIK Group of Companies. Xience is a trademark or registered trademark of the Abbott Group of Companies.
© 2022 BIOTRONIK AG – All rights reserved.
Specifications are subject to modification, revision and improvement.