BIO-RESORT
36-month primary endpoint results RCT Orsiro and Synergy vs. Resolute Integrity
ORSIRO'S EXTENSIVE CLINICAL PROGRAM
including BIO-RESORT
Conclusions
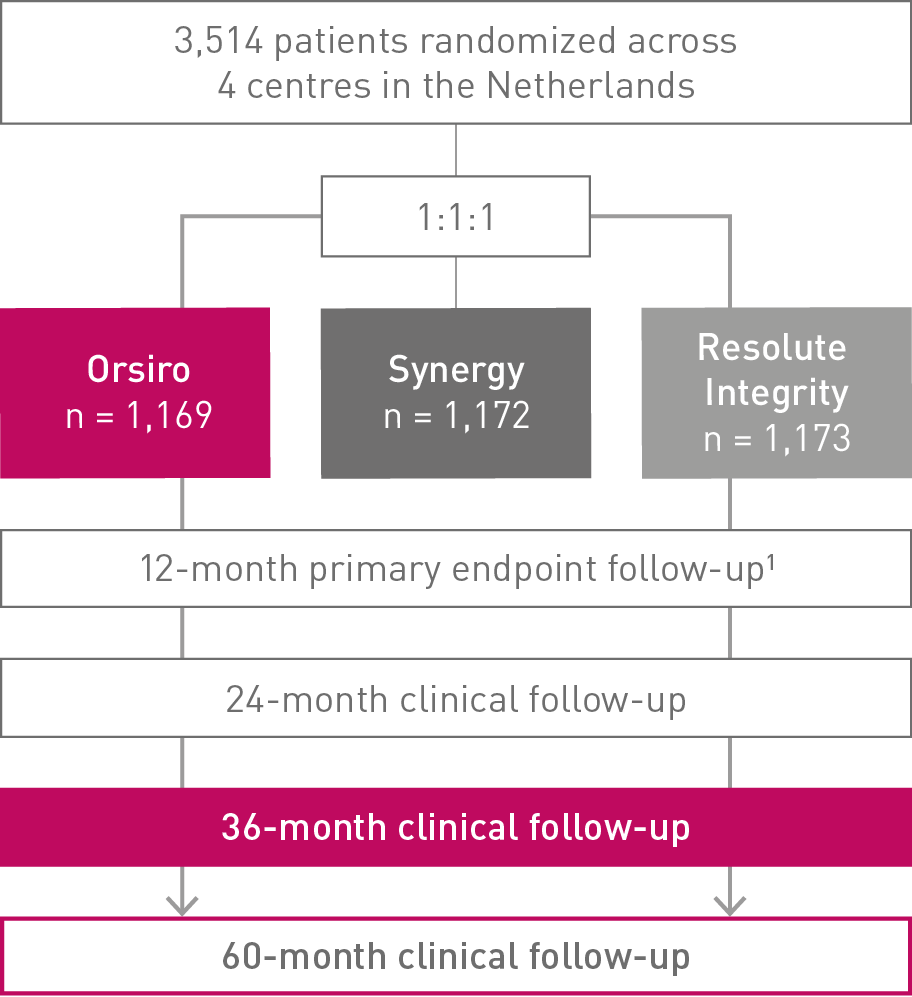
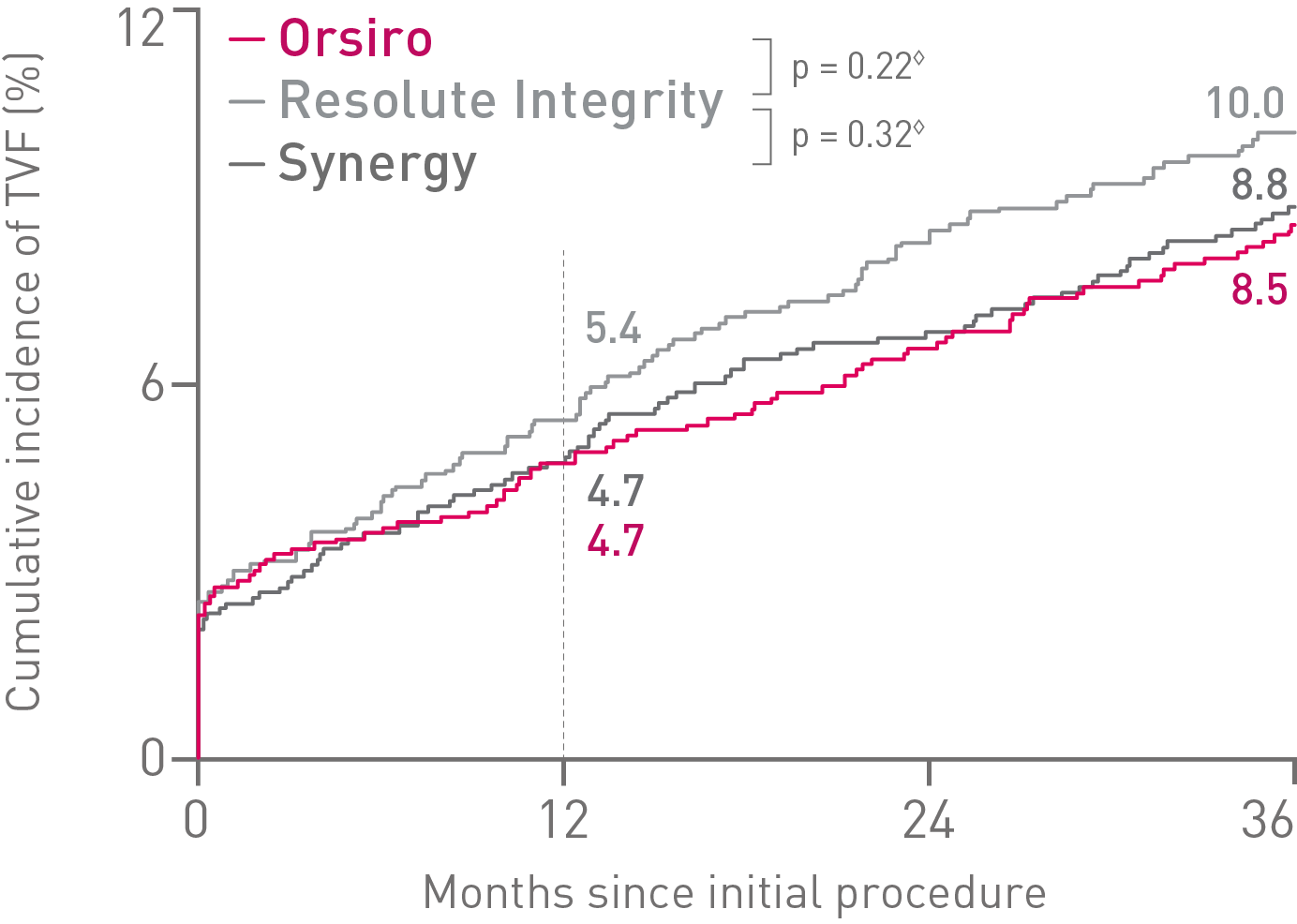
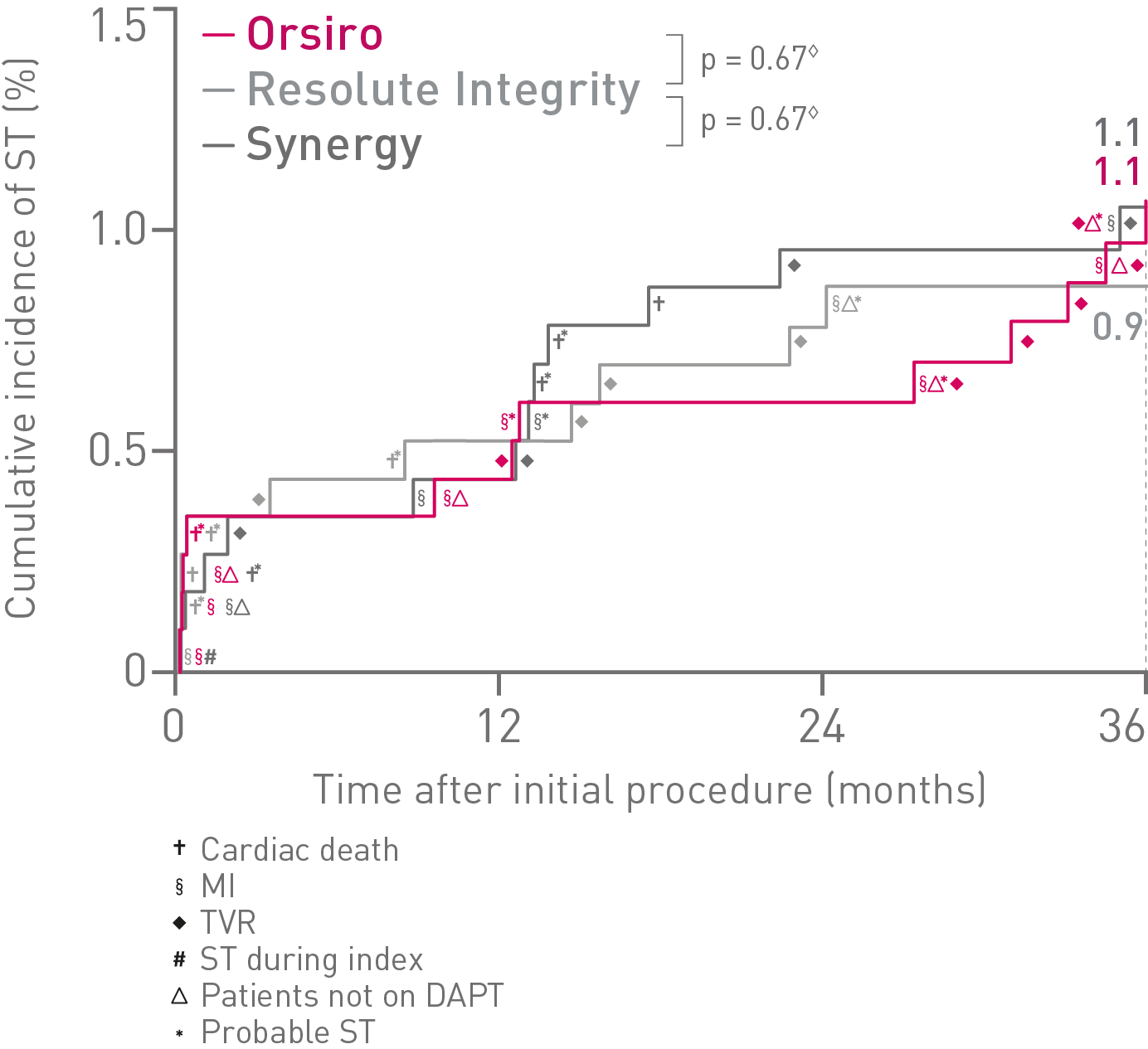
• In this 3,514 patient large, randomized, investigator-initiated, all-comers trial, Orsiro® demonstrated non-inferiority to Resolute Integrity* while performing equally well as Synergy** (primary endpoint Target Vessel Failure (TVF) at 12 months: Orsiro 4.7%, Synergy 4.7%, Resolute Integrity 5.4%, pnon-inferiority < 0.0001).
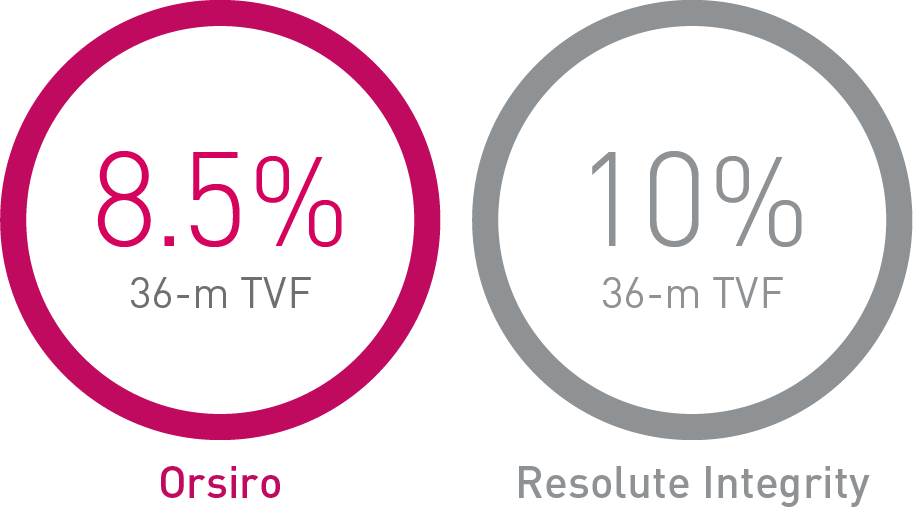
• At 36 months, in this highly complex patient population, Orsiro has shown favorable outcomes with numerically lower event rates in TVF compared to both Synergy and Resolute Integrity.
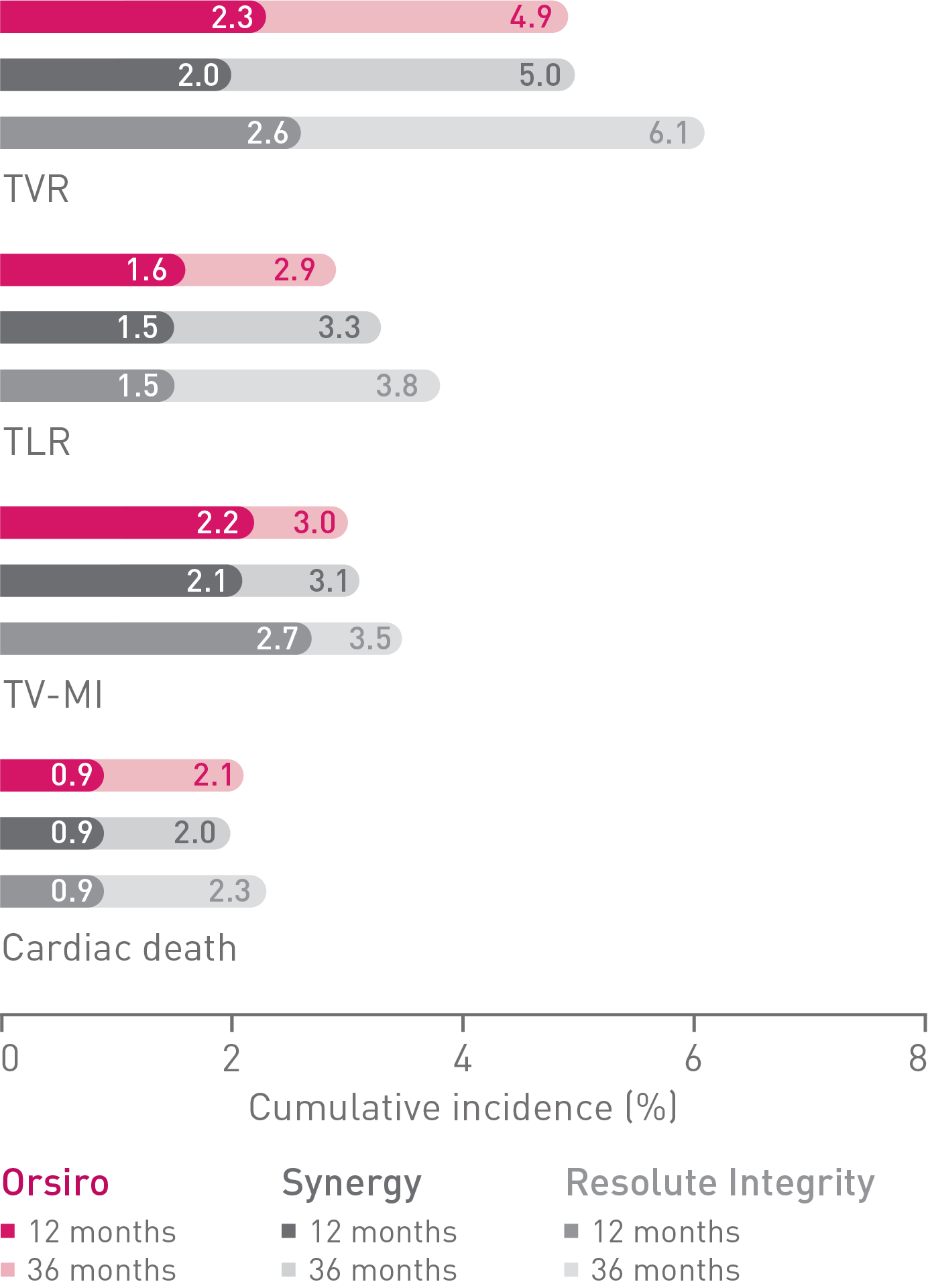
• Additionally, there were no differences observed between Orsiro and Synergy when compared to Resolute Integrity for safety endpoints