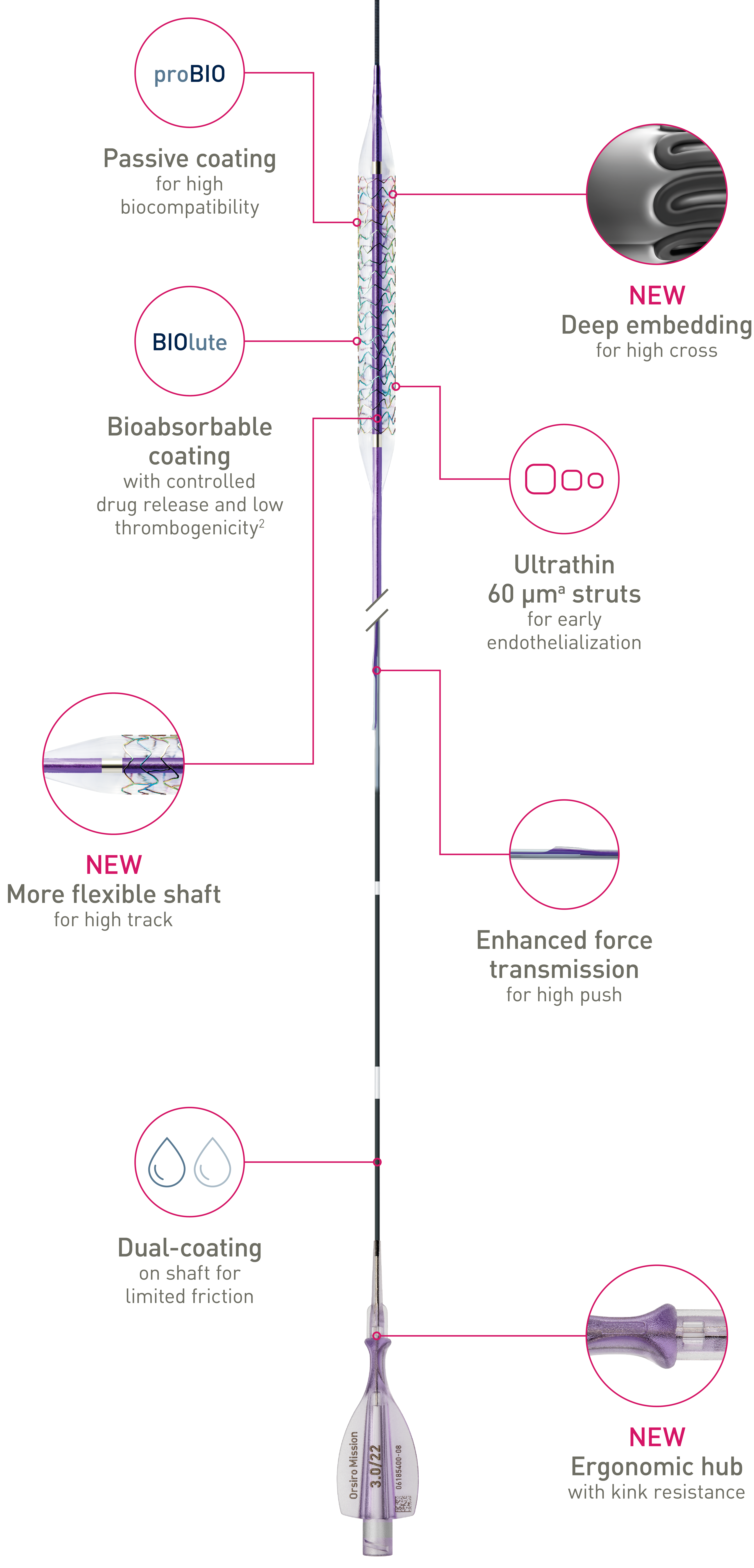
The next level of deliverability1
The next level of deliverability1
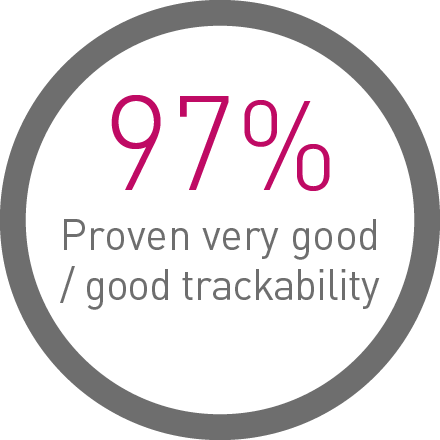
1st in Push3
Transmitting up to 96% more force from hub to tip.

Proven deliverability on the bench and in a real-world user evaluation of over 1,000 implantations:4
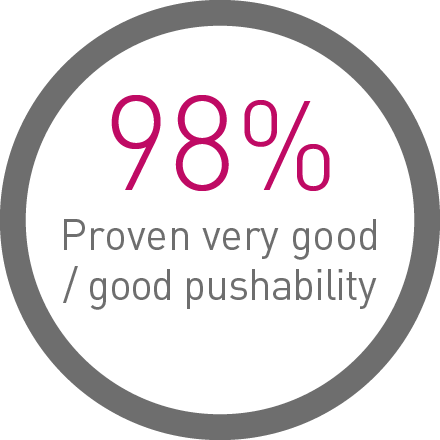
Proven deliverability on the bench and in a real-world user evaluation of over 1,000 implantations:4

1st in Track3
Up to 33% less force needed to follow the path to the lesion.
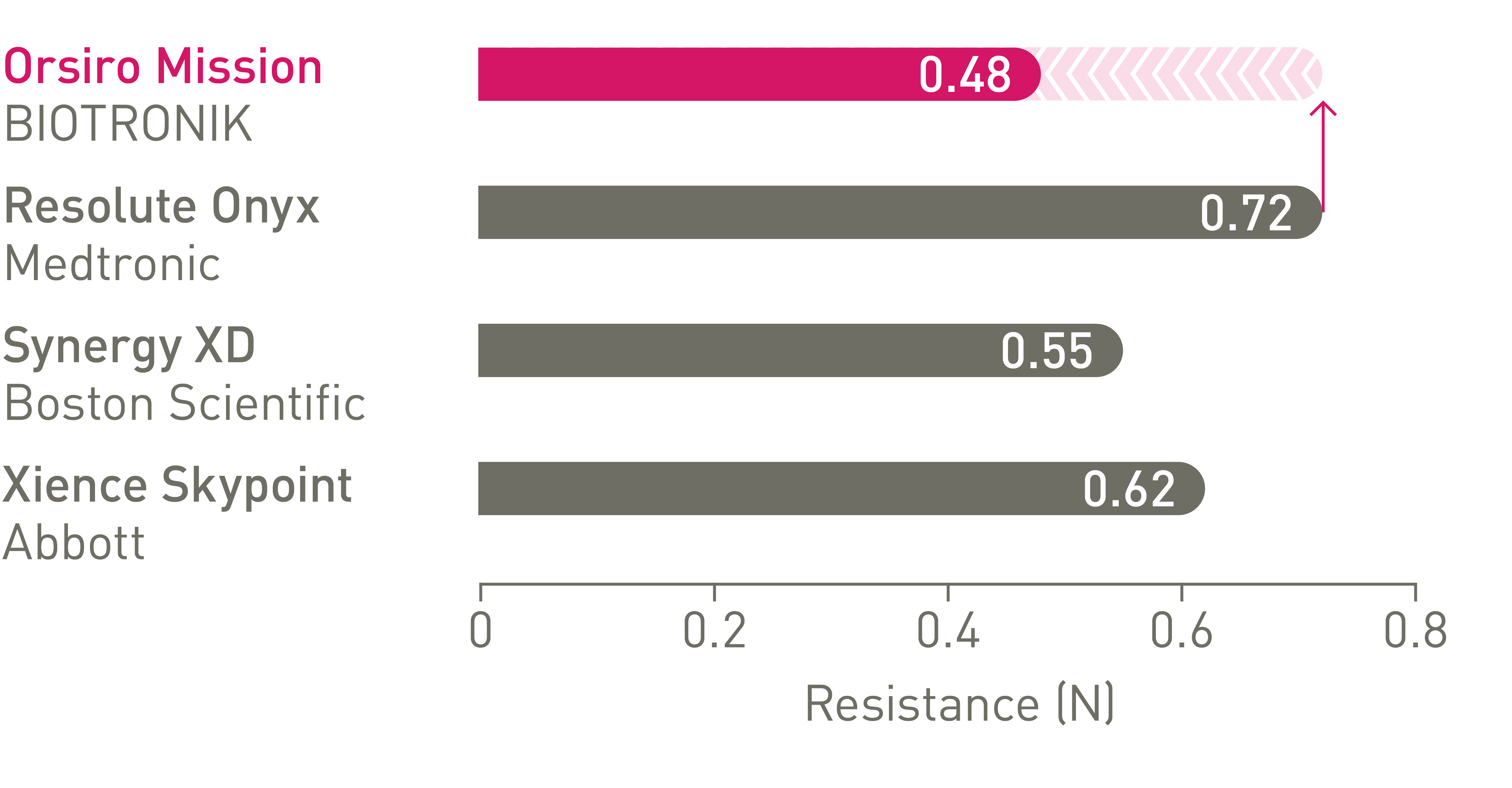

1st in Cross3
Up to 64% less force needed to successfully cross demanding anatomies.

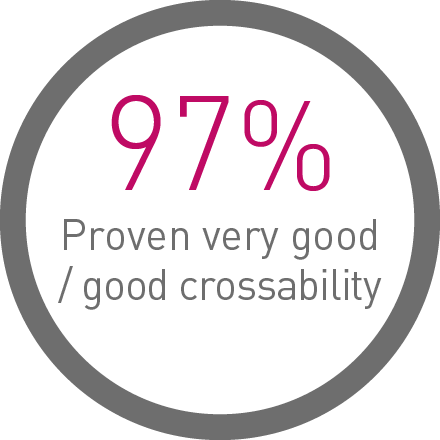



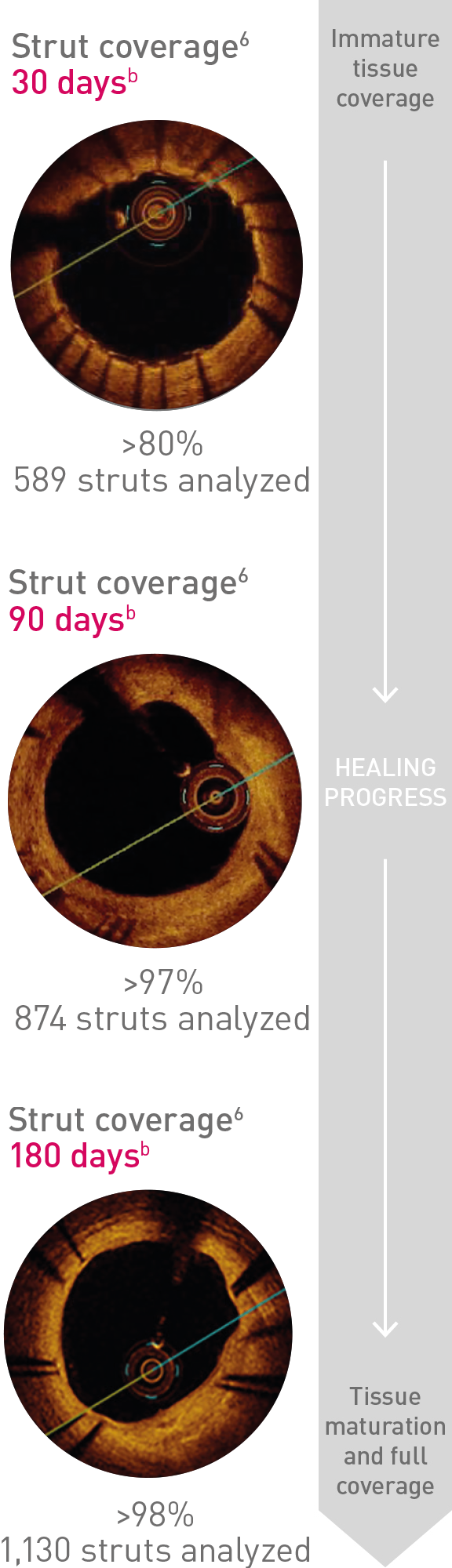
Ultrathin struts5
Ultrathin struts5
For early endothelialization

Strut thickness in perspective7

CoCr-SES, 60 μma

PtCr-EES, 74 μm

CoCr-SES, 80 μm

CoNi-ZES, 81 μm

CoCr-EEs, 81 μm

PtCr-EES, 81 μm

316L-BES, 120 μm
Outstanding patient outcomes10
Outstanding patient outcomes10
One of the most studied DES11
>
55,000
patients enrolled12
>
71,500
patients enrolled or
planned in total12
planned in total12
>
68
studies started12
Orsiro – the highest probability (70.8%) to rank as the best stentc
Taglieri et al. network meta-analysis (n = 99,039 patients, 77 RCTs)13

“
If we want to inform our clinical practice on the best evidence available, we have to acknowledge that at 1-year the best stent, is the Orsiro stent.”
Dr. Tullio Palmerini,
Policlinico S. Orsola, Malpighi,
Bologna, Italy
Pushing the boundaries of safety performance with Orsiroe
BIOFLOW-V (n = 1,334), 5-Year results of the FDA pivotal trial14

Orsiro Mission DES is indicated for complex patients and lesionsh
Orsiro Mission DES is indicated for complex patients and lesionsh
ACS
STEMI
DM
HBR
B2C
SV
MVD
Continued Superiority in STEMI at 2 years15,i
BIOSTEMI (n=1,300)
Continued Superiority in STEMI at 2 years15,i
BIOSTEMI (n=1,300)
BIOSTEMI (n=1,300)
5.1%
Orsiro
8.1%
XIENCE
Target Lesion Failure (TLF) rate at 2 years.
Rate Ratio (95% BCIj): 0.58 (0.40-0.84) Posterior probability of Superiority: 99.8%
Bayesian ITT Populationk

Low stent thrombosis (ST) at 5 years16
BIO-RESORT Small Vessels (n=1,506)
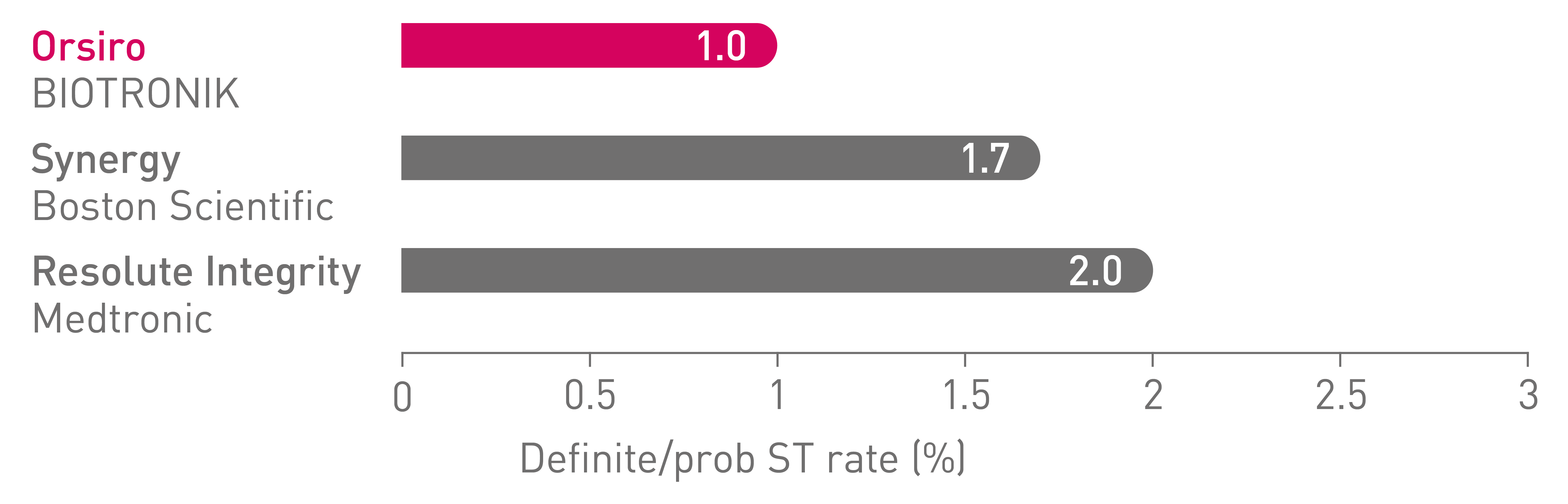
Low stent thrombosis (ST) at 5 years16
BIO-RESORT Small Vessels (n=1,506)
BIO-RESORT Small Vessels (n=1,506)