Principal investigator of the BIOSTEMI-ES Trial presents 5-year results
Juan F. Iglesias, MD, Geneva, Switzerland
Principal investigator of the BIOSTEMI-ES Trial presents 5-year results
Juan F. Iglesias, MD, Geneva, Switzerland
Proven superiorityb,2
Proven superiorityb,2
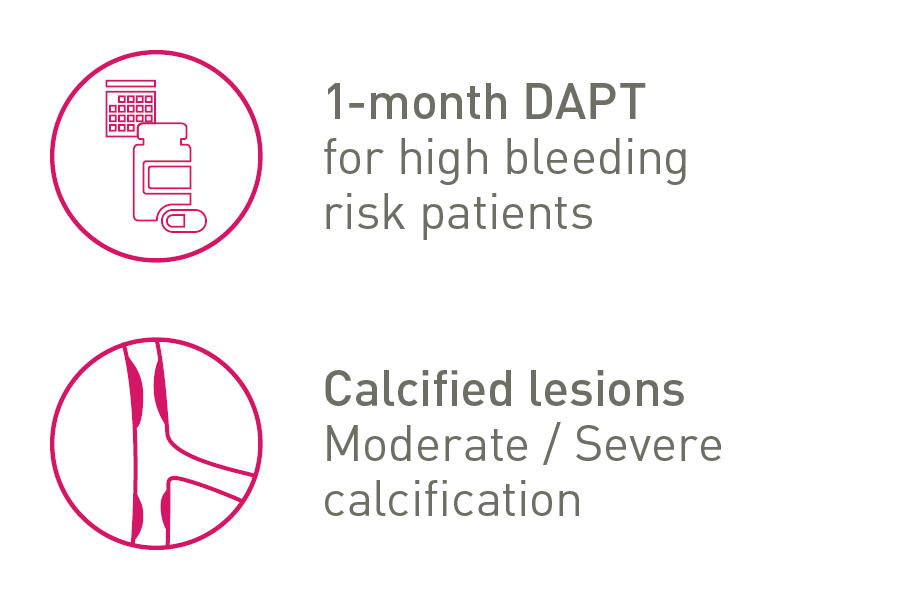
NEW INDICATIONS
CE-approved indications provide shortened dual antiplatelet therapy (DAPT) for high bleeding risk patients and treatment for moderate to severe calcified lesions
STEMI = ST-Elevation Myocardial Infarction
a. BIOTRONIK data on file (n = 5), based on statistically significant differences on the bench for Pushability, Trackability, and Crossability compared to Xience Skypoint, superior to Xience in STEMI patients; b. Clinical data collected with the Orsiro DES device within the Orsiro DES family clinical program; c. Based on target lesion failure at 5-year follow-up; d. Clinical data collected with the Orsiro Mission DES device within the Orsiro DES family clinical program. d. Clinical data collected with the Orsiro Mission DES device within the Orsiro DES family clinical program
1. Iglesias JF. et al, Long-term outcomes with biodegradable polymer sirolimus eluting stents versus durable polymer everolimus-eluting stents in ST-segment elevation myocardial infarction: 5-year follow-up of the BIOSTEMI randomized superiority trial, The Lancet, 2024; 2. In large RCTs, based on Taglieri et al. Meta-analysis, against currently used DES; 2. Iglesias J.F., BIOSTEMI 5 year follow up, Presented at TCT 2023, San Francisco USA.
Orsiro, Orsiro Mission, proBIO and BIOlute are trademarks or registered trademarks of the BIOTRONIK Group of Companies. All other trademarks are the property of their respective owners
© 2025 BIOTRONIK AG – All rights reserved.
Specifications are subject to modification, revision and improvement.

