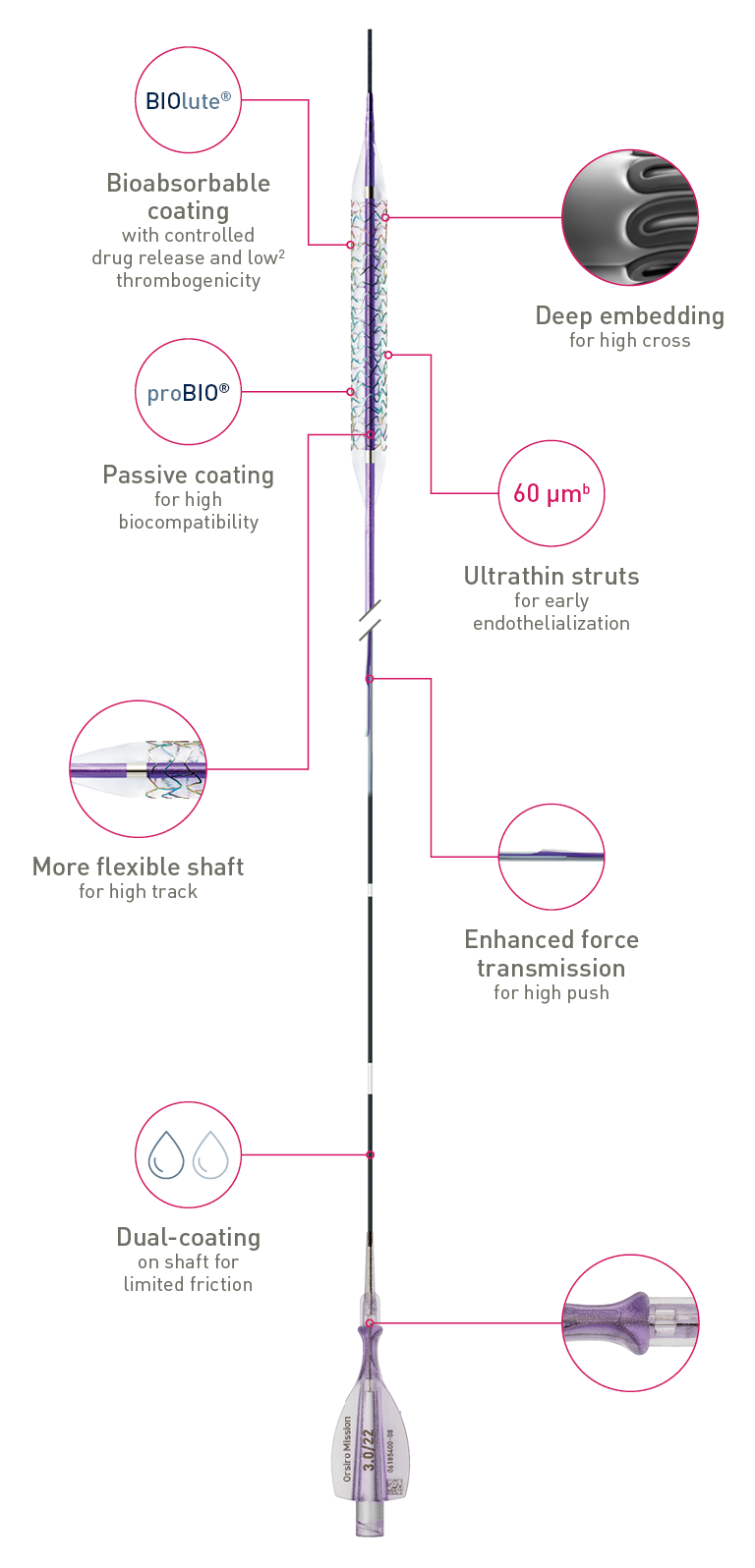
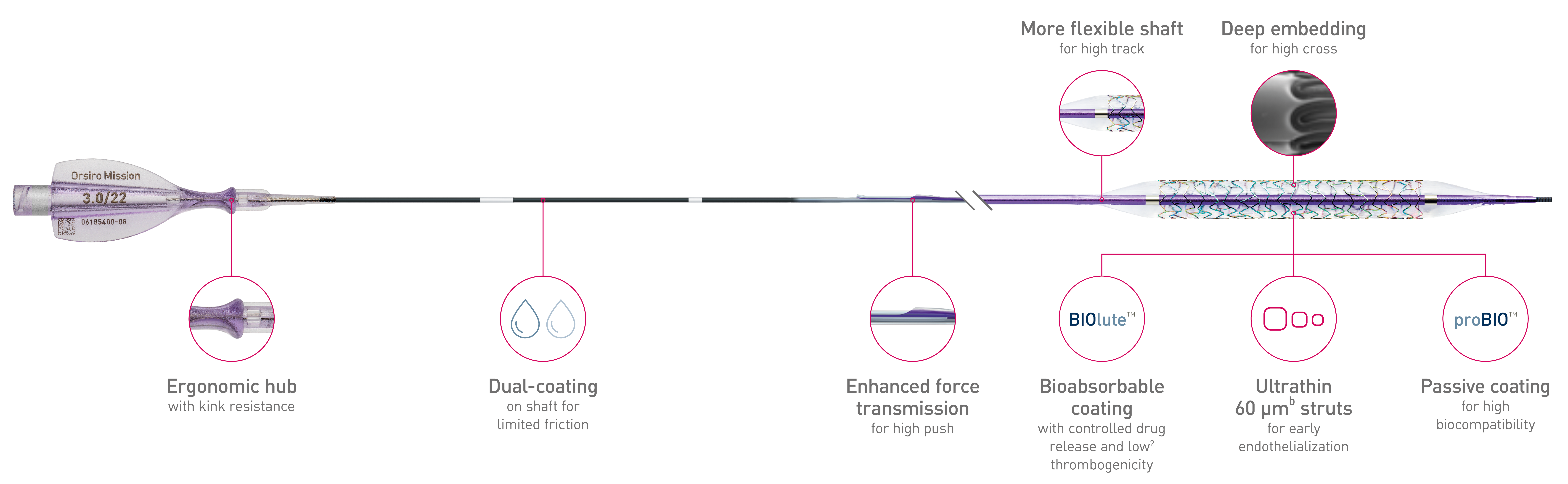
The next level of deliverability2
The next level of deliverability1
Better pushability3
Transmitting up to 96% more force from hub to tip.



Better trackability3
Up to 33% less force needed to follow the path to the lesion.
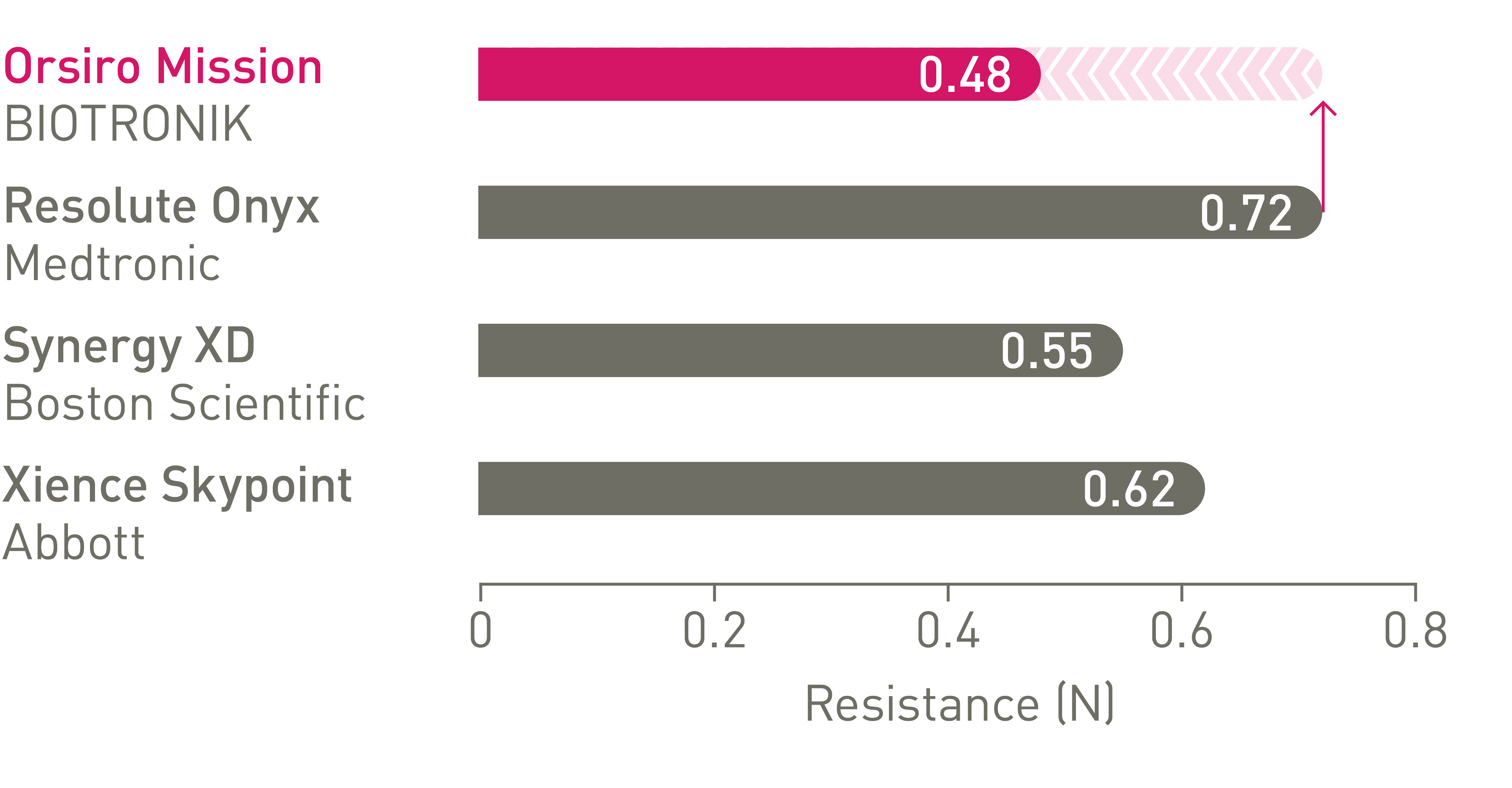


Better crossability3
Up to 64% less force needed to successfully cross demanding anatomies.





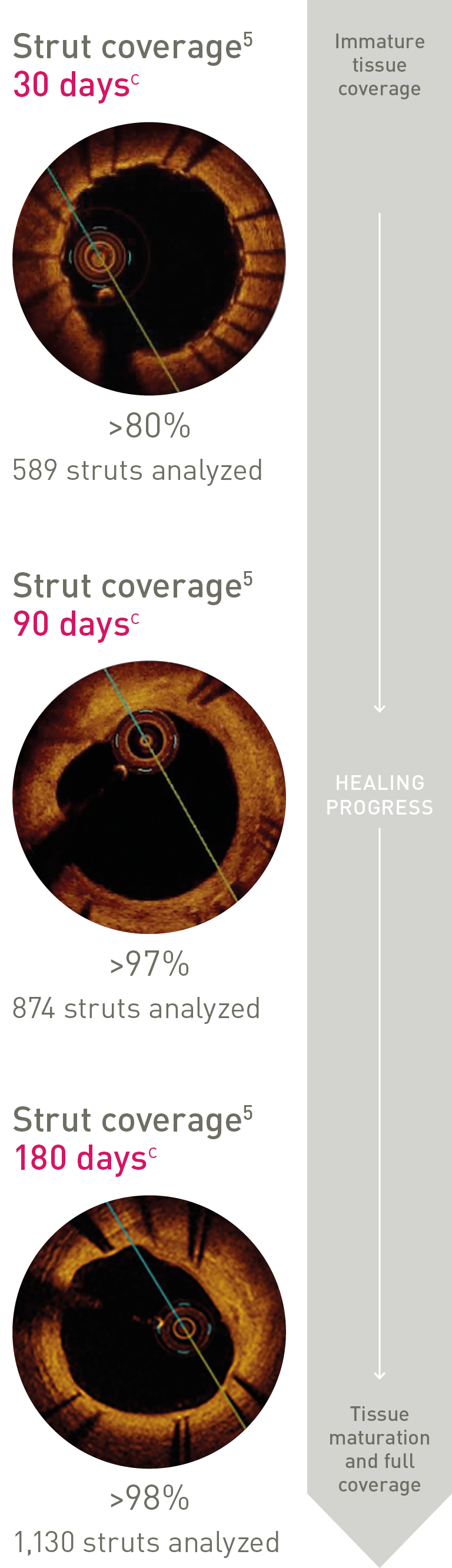
Ultrathin struts4
Ultrathin struts5
For early endothelialization
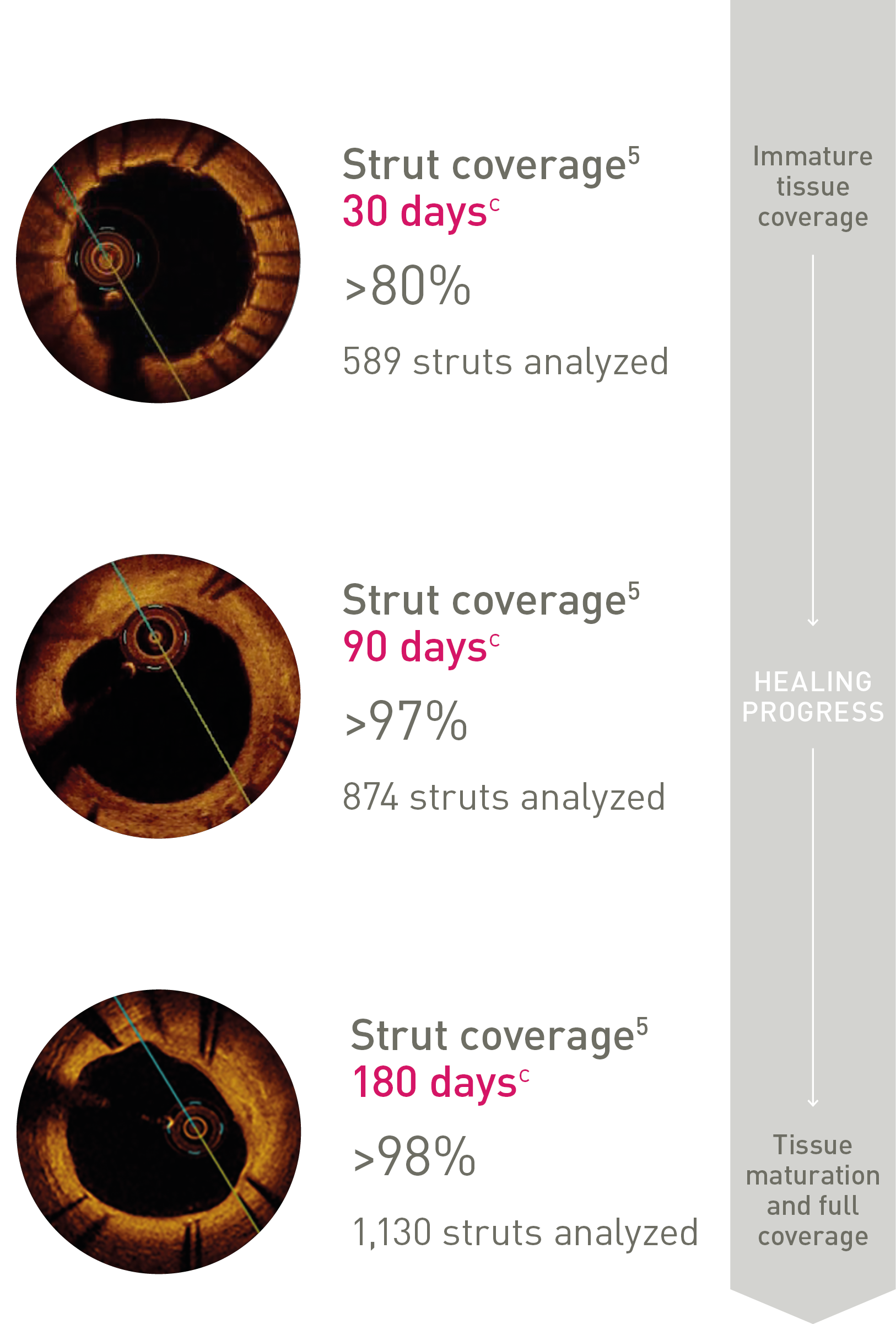
Strut thickness in perspective6

CoCr-SES, 60 μmb

PtCr-EES, 74 μm

CoCr-SES, 80 μm

CoNi-ZES, 81 μm

CoCr-EEs, 81 μm

PtCr-EES, 81 μm

316L-BES, 120 μm
Outstanding patient outcomes9,d
Outstanding patient outcomes10
Orsiro familyof DES - One of the most studied DES10,d,e
>
100,000
Patients enrolled or
planned in total 11,d,e
planned in total 11,d,e
>
71,000
Patients enrolled or
planned in total11,d,e
planned in total11,d,e
>
86
studies started11,d,e

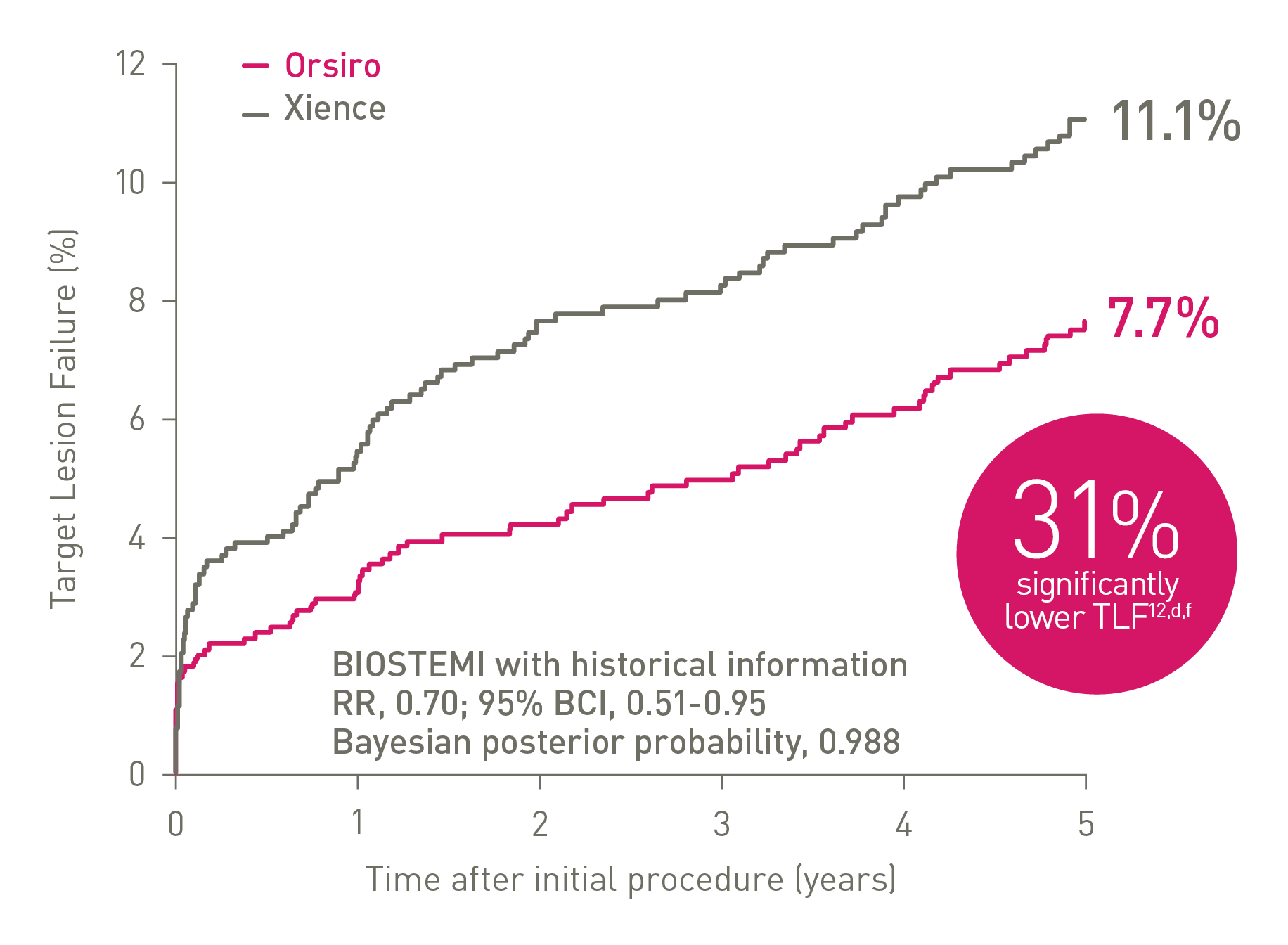
TLF at 5 years - Continued superiority in STEMI12,d
Orsiro Mission DES is indicated for complex patients and lesionsg
Orsiro Mission DES is indicated for complex patients and lesionsg
DM
ACS
SV
B2/C
Calcified lesionsi
HBR
MVD
STEMI
1-month DAPTi
Proven superiority in STEMI12,d,h
Proven superiority in STEMI12,d,h
Proven safety and efficacy for 1-month DAPT13,i,d
![]()
Proven safety and efficacy for 1-month DAPT13,i,d