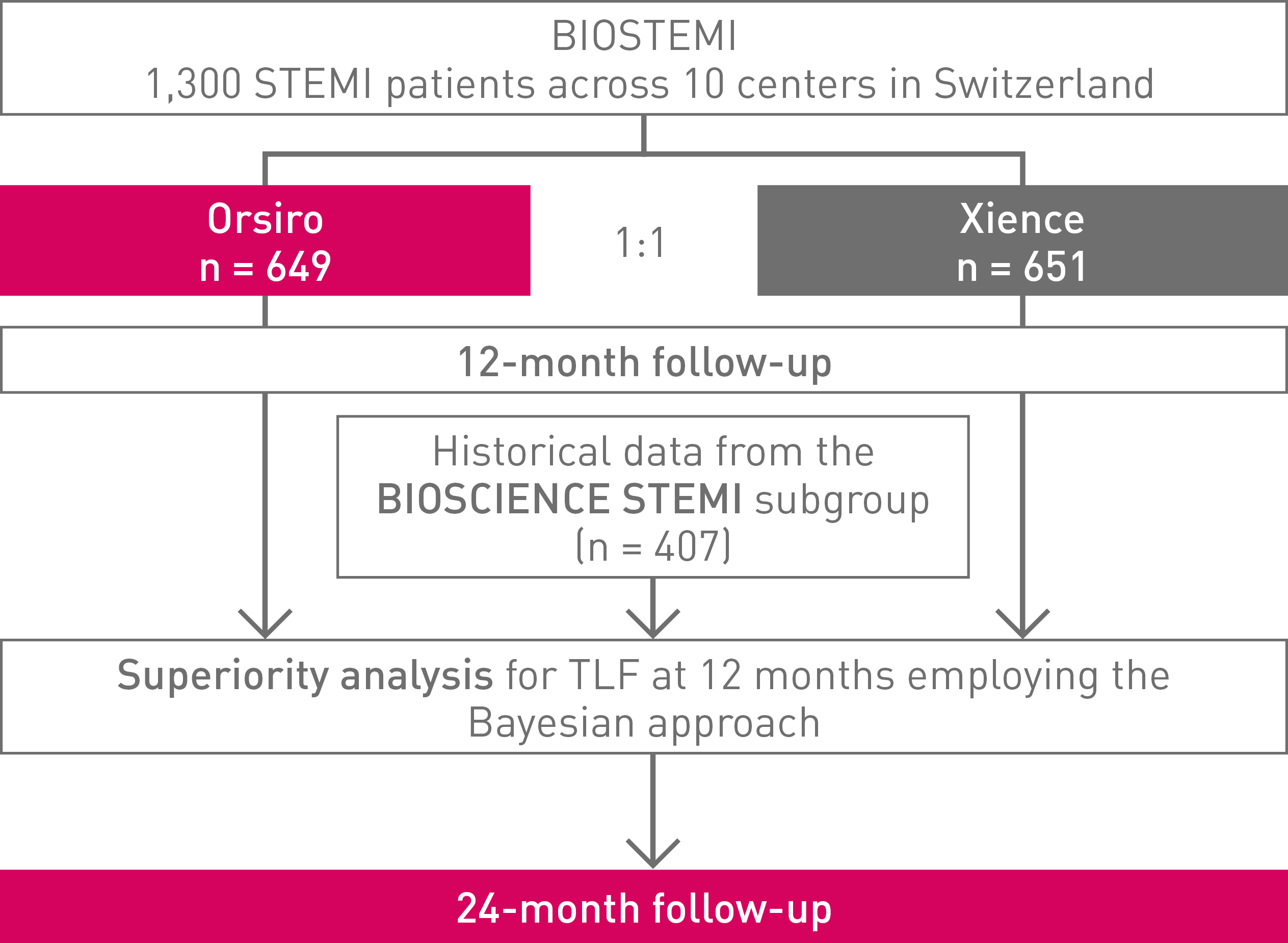
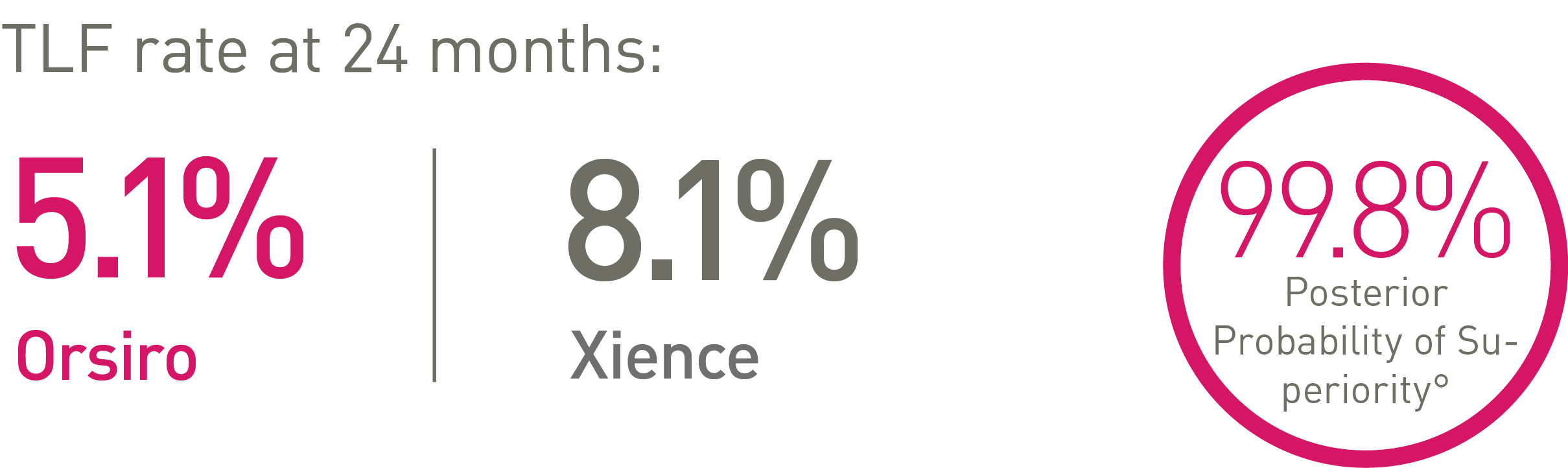BIOSTEMI
Biodegradable polymer sirolimus-eluting stent Orsiro® versus Durable Polymer everolimus-eluting stent Xience in patients with ST-segment Elevation Myocardial Infarction (STEMI) at 24 months
BIOSTEMI trial: Continued superiority in STEMI at 2 years°
Dr. Juan F. Iglesias, Geneva University Hospitals, Geneva, Switzerland presents the BIOSTEMI 2-year results
Conclusions
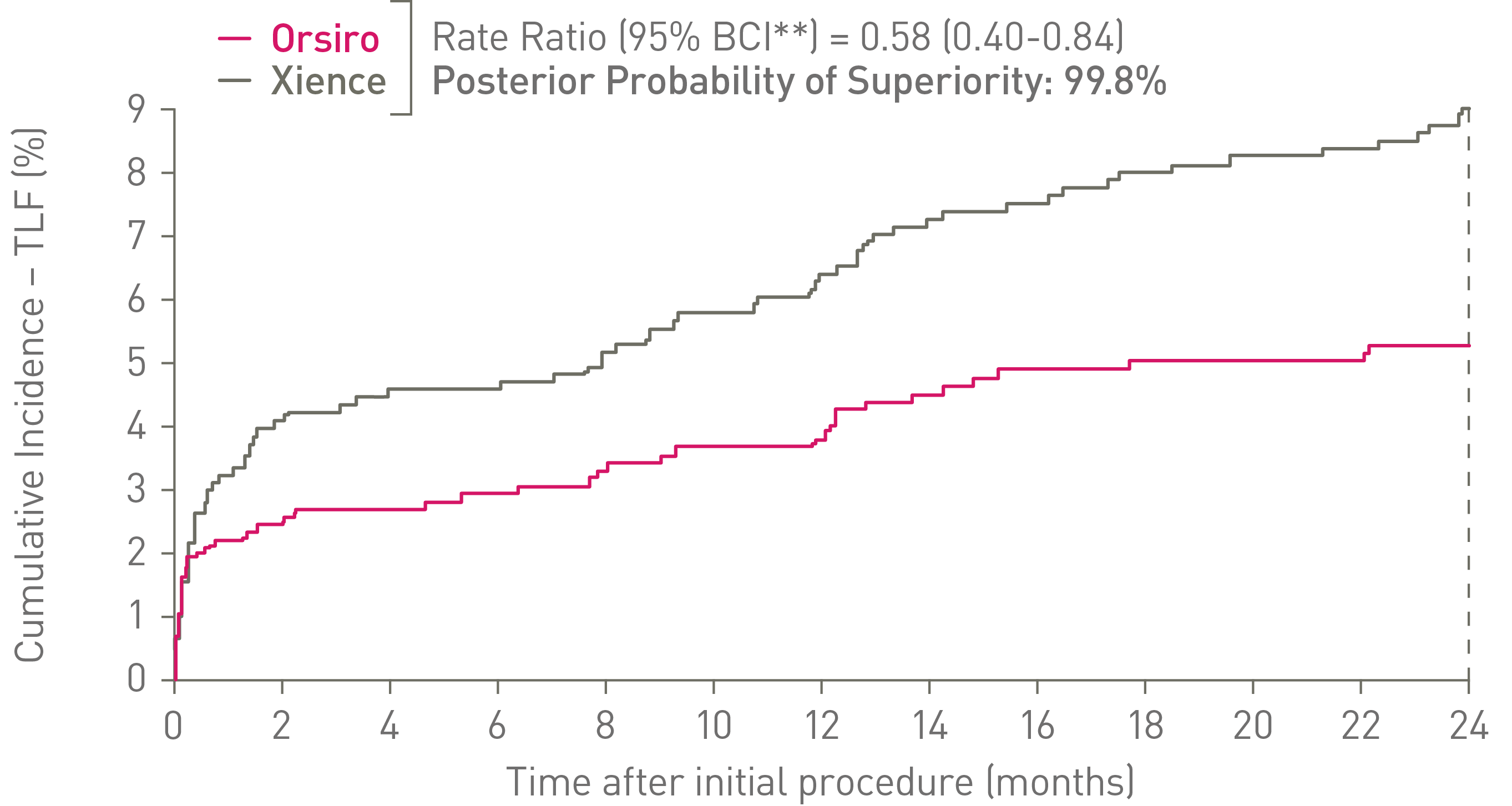
• At 24 months, Orsiro is superior to Xience in STEMI patients with respect to the primary endpoint of Target Lesion Failure (TLF) (5.1% vs. 8.1%, Rate Ratio (95% BCI**): 0.58 (0.40-0.84), Posterior Probability of Superiority: 99.8%)
• The difference in TLF rates remained statistically significant after the exclusion of historical information from the BIOSCIENCE trial (Rate Ratio (95% BCI**): 0.62 (0.40-0.96), Posterior Probability of Superiority: 98.5%)
• Clinically-indicated Target Lesion Revascularization (TLR) rate was significantly lower in Orsiro compared to Xience (2.5% vs. 5.1%, Rate Ratio (95% BCI**): 0.52 (0.30-0.87), Posterior Probability of Superiority: 99.3%)
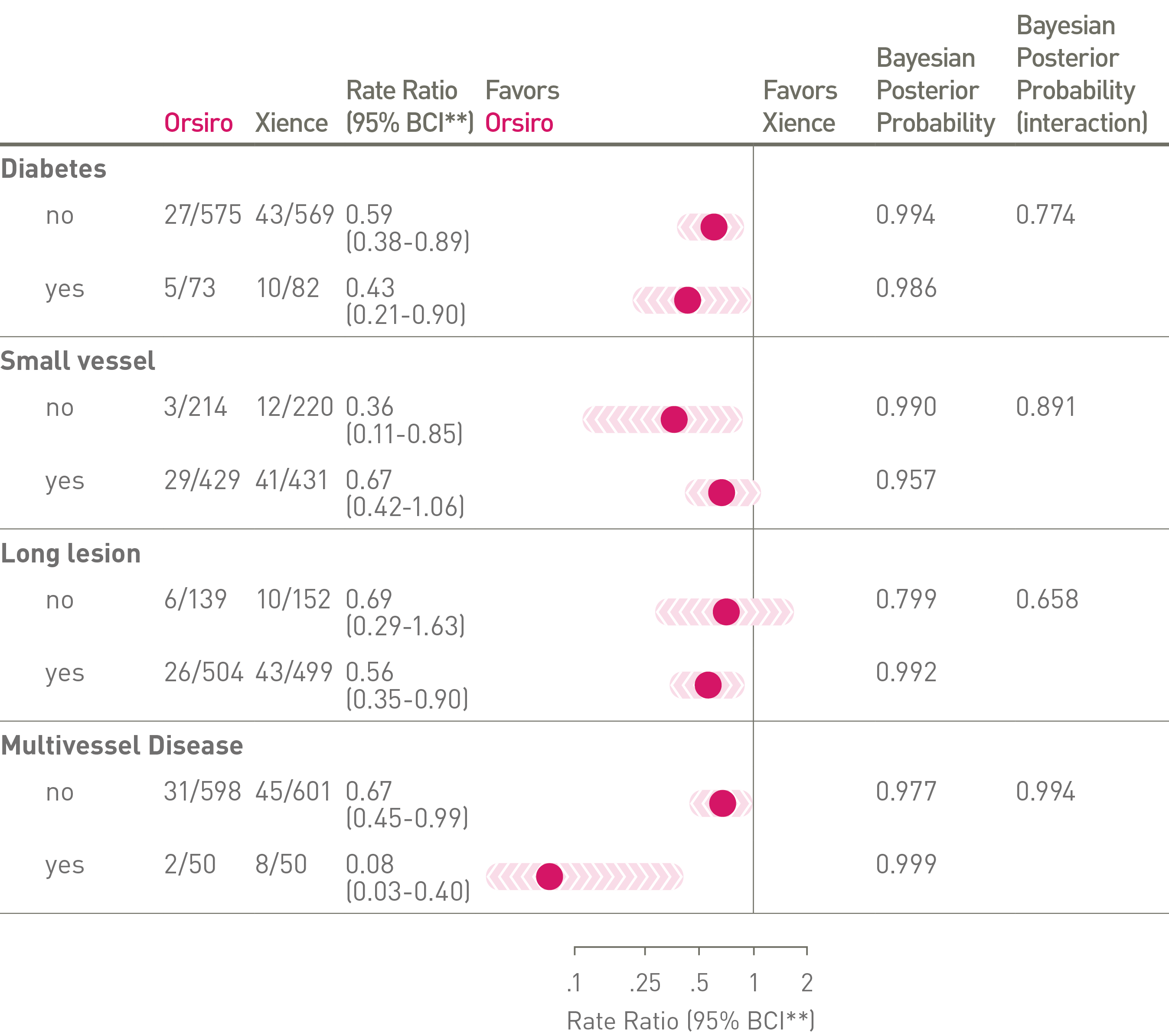
• The significant difference at 24-m favoring the Orsiro vs. Xience DES might have clinically relevant implications for routine clinical practice.