Full system
Full system
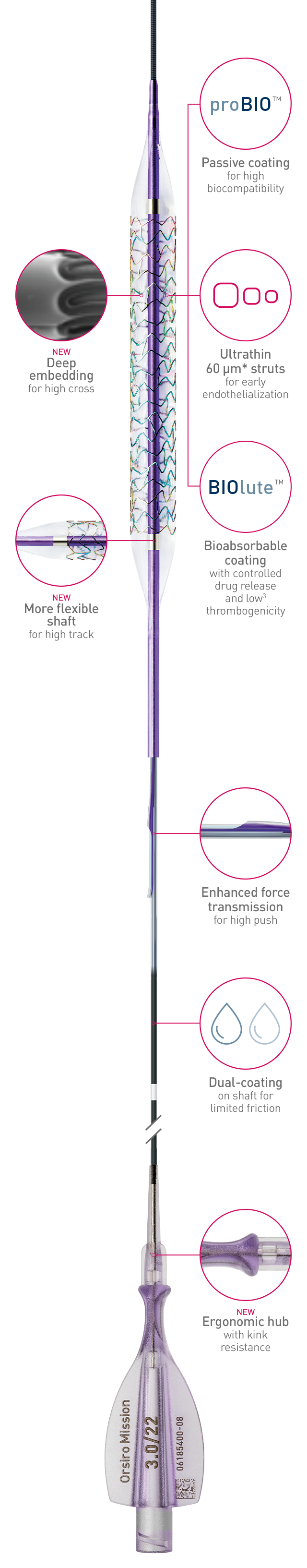
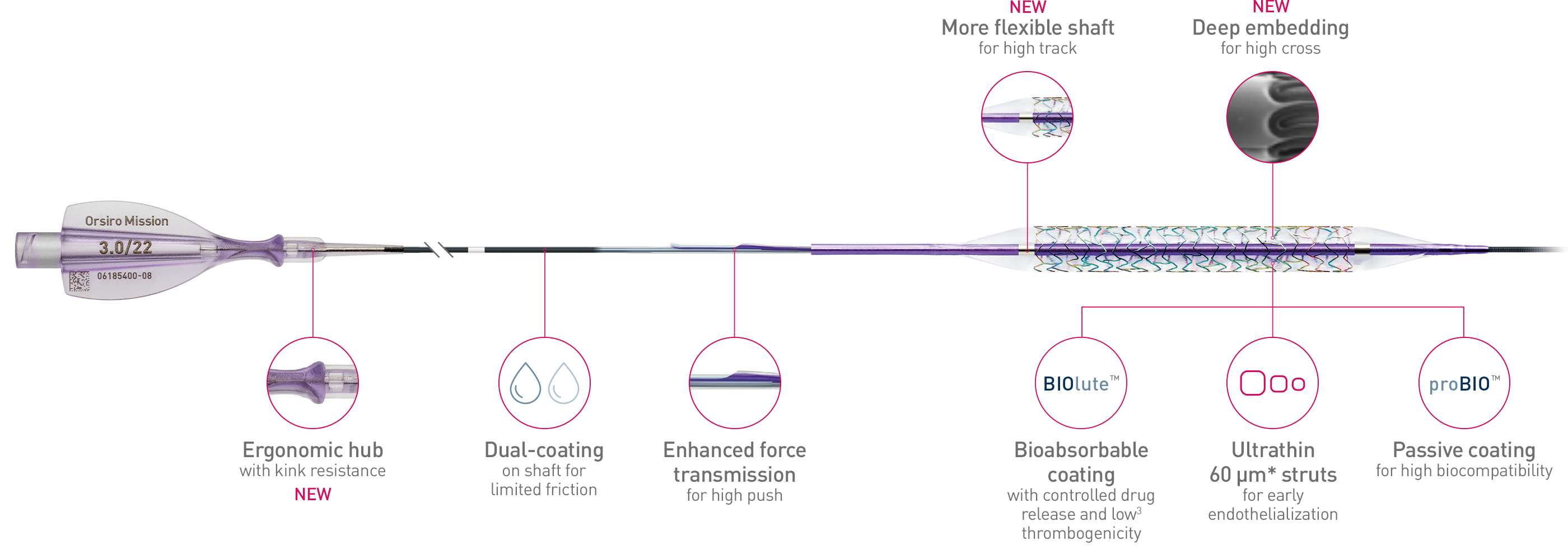
Full system


The next level of deliverability1
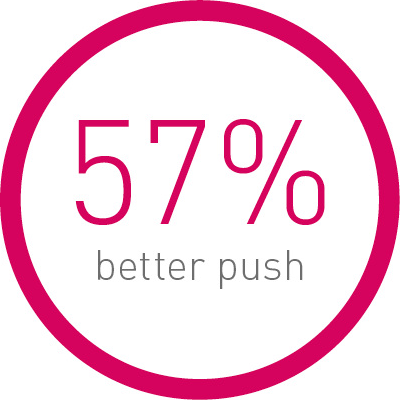
1st in Push2
Transmitting up to 57% more force from hub to tip.
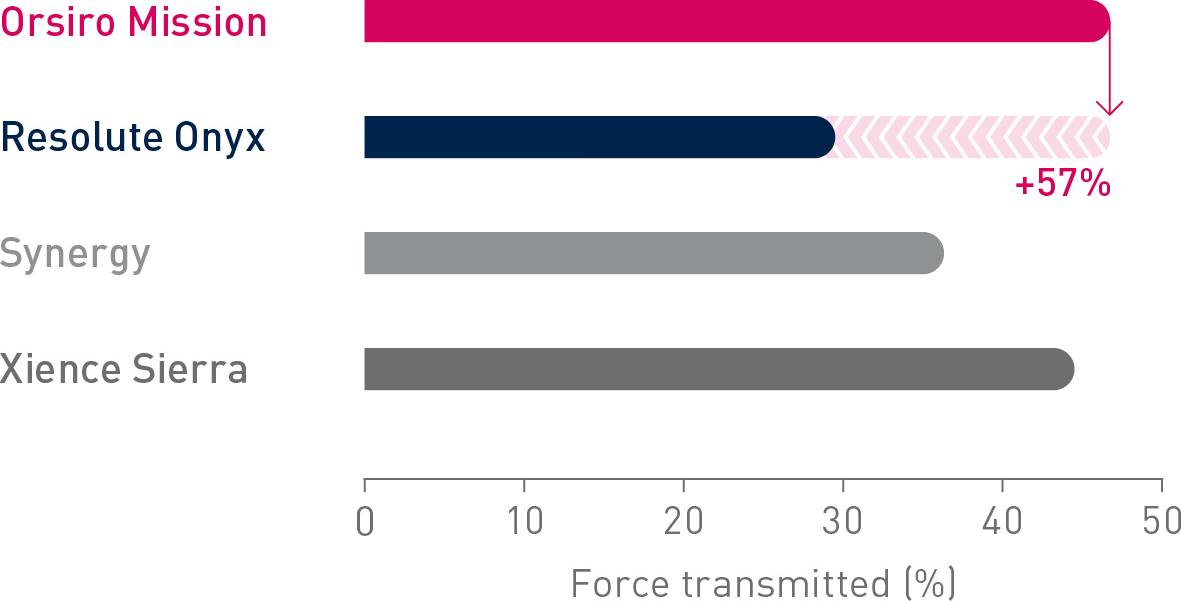
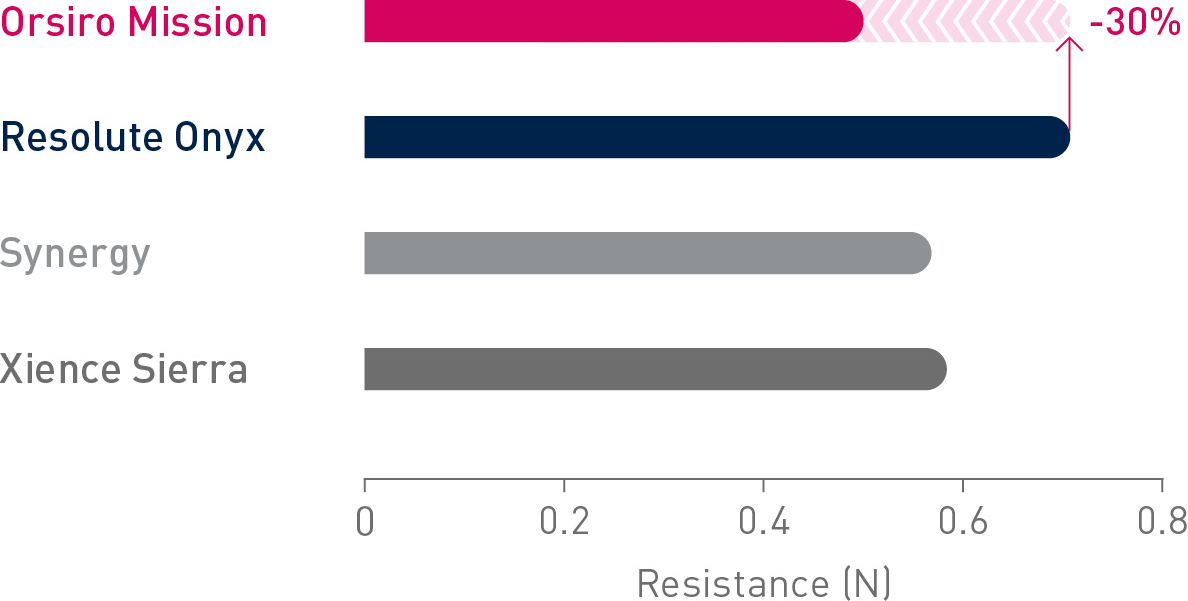
1st in Track2
Up to 30% less force needed to follow the path to the lesion.
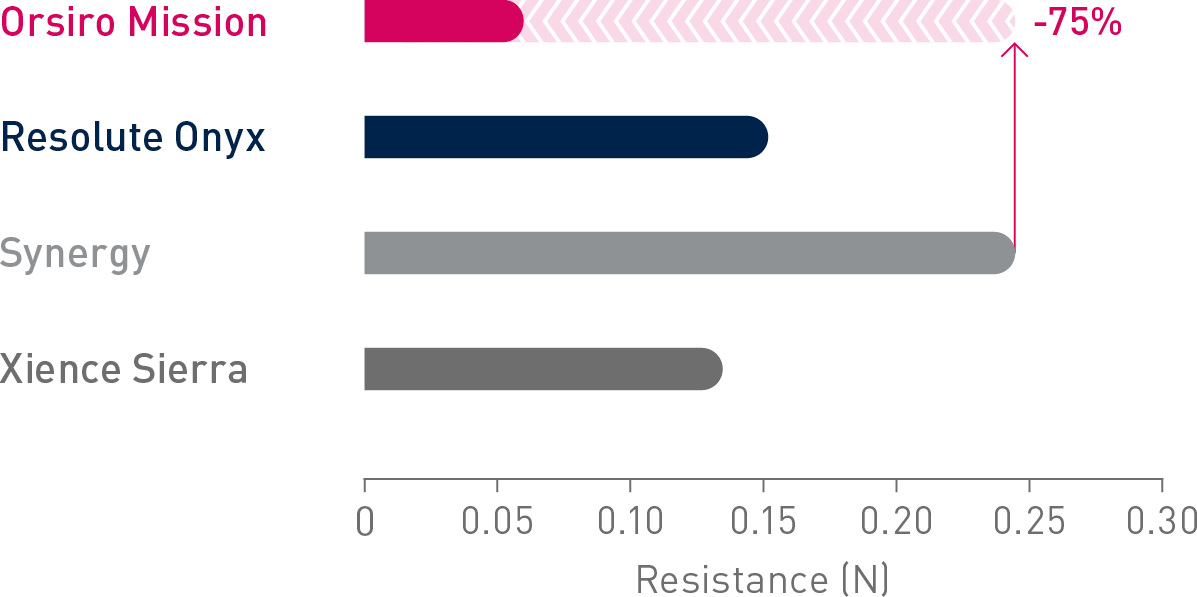
1st in Cross2
Up to 75% less force needed to successfully cross demanding anatomies.

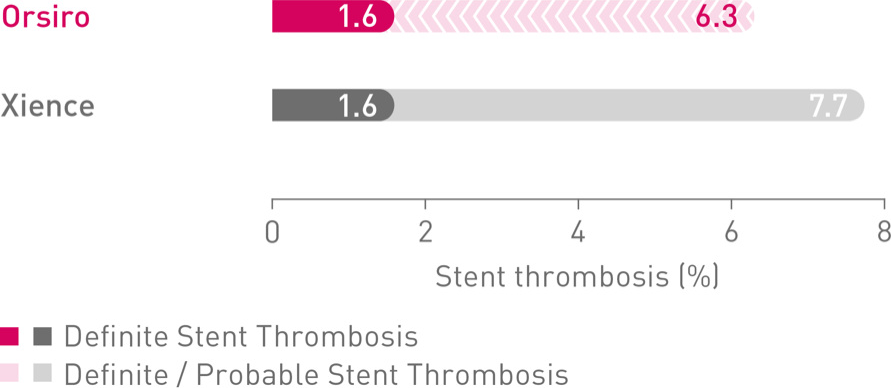
When a unique design translates into clinical benefits


Low definite Stent Thrombosis (ST) out to 5 years, BIOSCIENCE, all-comers RCT (n= 2,119)8
(n = 2,119 patients)


strut thickness in perspective5

CoCr-SES, 60 μm*
60 μm*

PtCr-EES, 74 μm
74 μm

CoCr-SES, 80 μm
80 μm

CoNi-ZES, 81 μm
81 μm

CoCr-EEs, 81 μm
81 μm

PtCr-EES, 81 μm
81 μm

316L-BES, 120 μm
120 μm
* ø 2.25 – 3.0 mm
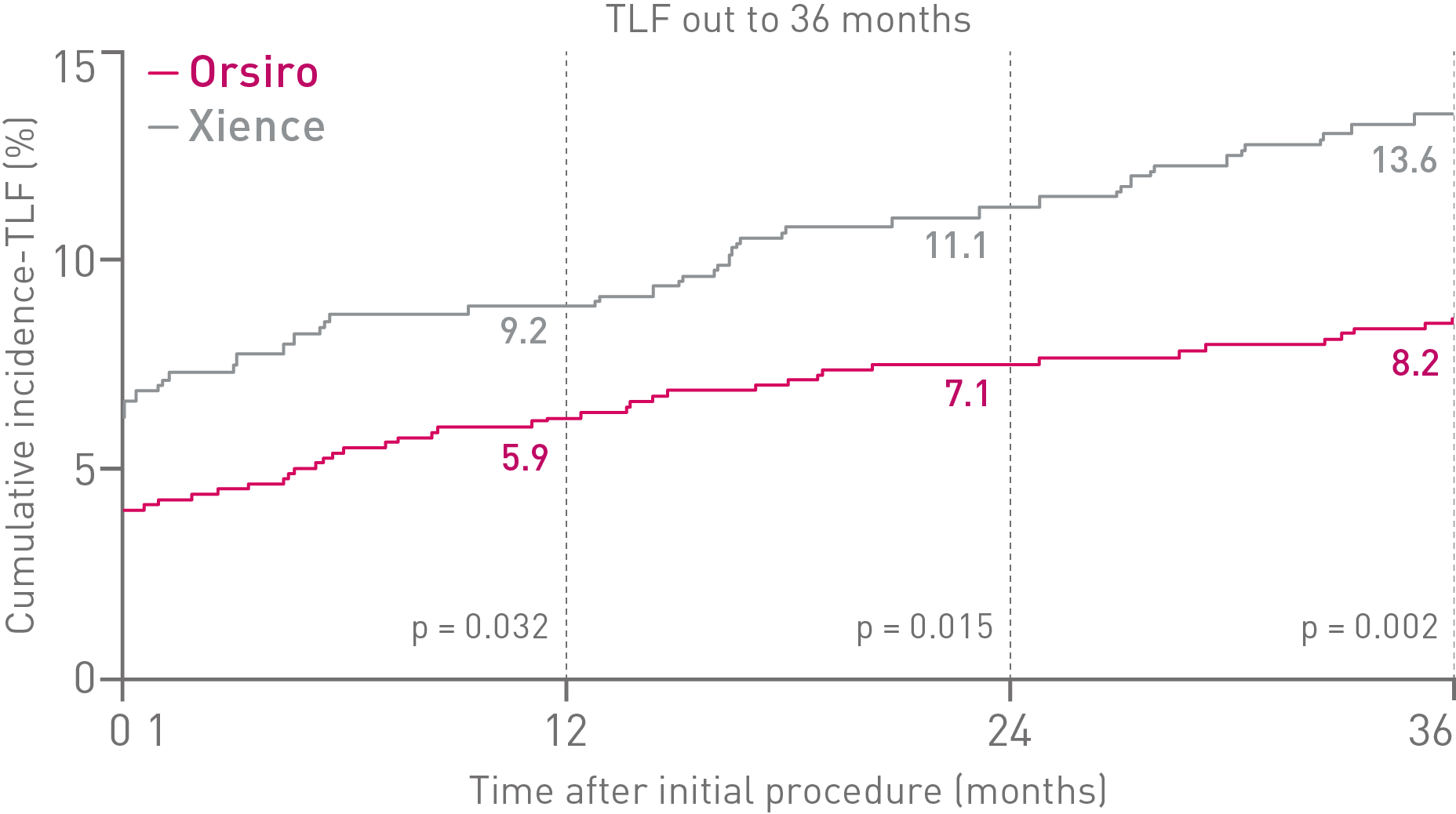
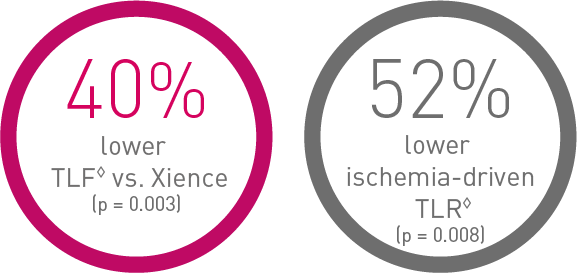
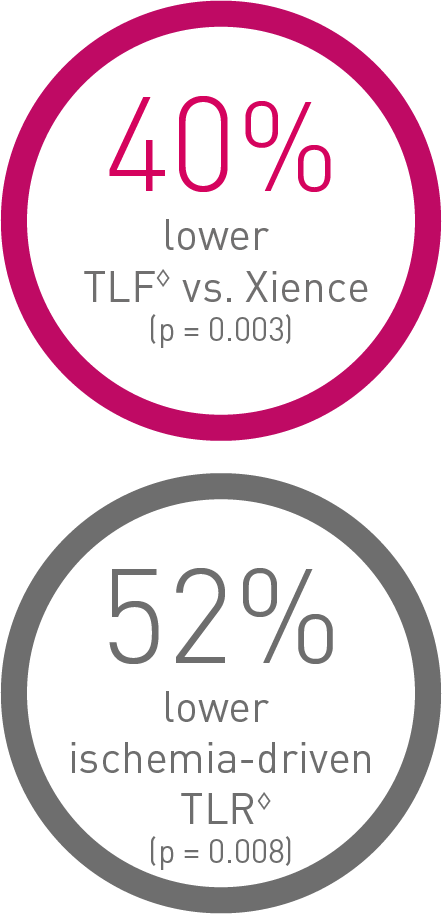
Outstanding patient outcomes10
Oustanding Orsiro DES11, 12, 13, 14
BIOFLOW-V, FDA pivotal trial (n = 1,334)

* ø 2.25 – 3.0 mm
∆ Images: Secco G et al. Time-related changes in neointimal tissue coverage following a new generation SES implantation: an OCT observational study. Presented at: euro PCR, May 20, 2014; Paris, France.
◊ Based on 36-m frequentist analysis.
§ As per IFU: ACS – Acute Coronary Syndrome; STEMI – ST-Elevation Myocardial Infarction; DM – Diabetes Mellitus; HBR – High Bleeding Risk; B2C – Complex Lesions; SV – Small Vessels; MVD – Multi-Vessel Disease.
** BCI: Bayesian Credibility Interval.
¤ n= 1,300 newly enrolled STEMI patients including 407 patients from the BIOSCIENCE STEMI subgroup used as prior information.
‡ Based on a Rate Ratio of 0.59.
1. In comparison to Xience Sierra, Resolute Onyx and Synergy for bench tests on pushability, trackability and crossability, BIOTRONIK data on file; 2. BIOTRONIK data on file; 3. Per investigators’ interpretation of preclinical studies with Orsiro as mentioned in Cassese et al. J Thorac Dis 2018;10(2):688-692; 4. As characterized with respect to strut thickness in Bangalore et al. Meta-analysis; 5. Stefanini GG et al. Coronary stents: novel developments. Heart. 2014 Jul 1;100(13):1051-61; 6. Low AF. Stent platform for procedural success: Introducing the Continuous Sinusoidal & Core Wire Technologies. Presented at: AsiaPCR; 22-24 January, 2015; Singapore, Singapore; 7. Tolentino A. Evolving DES Strategy: Biodegradable Polymer vs. Bioabsorbable Scaffold. Presented at: Cardiovascular Nurse/Technologist Symposium; June 17, 2016; New York, USA; 8. Pilgrim T et al. 5-year outcomes of the BIOSCIENCE randomised trial. Supplementary appendix; Lancet 2018; published online Aug 28. http://dx.doi.org/10.1016/ S0140-6736(18)31715-X; 9. Secco G et al. Time-related changes in neointimal tissue coverage of a novel Sirolimus eluting stent: Serial observations with optical coherence tomography. Cardiovascular Revascularization Medicine 17.1 (2016): 38-43; 10. Based on investigator’s interpretation of BIOFLOW-V primary endpoint result; 11. Kandzari D, et al. BIOFLOW-V: A Prospective Randomized Multicenter Study to Assess the Safety and Effectiveness of the Orsiro Sirolimus Eluting Coronary Stent System in the Treatment Of Subjects With up to Three De Novo or Restenotic Coronary Artery Lesions Science. Presentation at E SC 2017; 12. Kandzari D et al. Ultrathin Bioresorbable Polymer Sirolimus-Eluting Stents versus Thin Durable Polymer Everolimus-Eluting Stents: Journal of American College of Cardiology (2018), doi: https //doi.org/10.1 016/j.jac c.2018. 09.019; 13. Kandzari D et al. J Am Coll Cardiol. Cardiovasc Interven. 2020, doi: 10.1016/ j.jcin.2020.02.019; 14. Kandzari D et al. J Am Coll Cardiol. Cardiovasc Interven. 2020. Supplemental Material; 15. Iglesias JF et al. Biodegradable polymer sirolimus-eluting stents versus durable polymer everolimus-eluting stents in patients with ST-segment elevation myocardial infarction (BIOSTEMI): a single-blind, prospective, randomised superiority trial; Lancet, September, 2019; 16. Buiten R et al. Outcomes in patients treated with thin-strut, very thin-strut, or ultrathin-strut drug-eluting stents in small coronary vessels – A prespecified analysis of the randomized BIO-RESORT trial; JAMA Cardiol. Published online May 21, 2019. doi:10.1001/jamacardio.2019.1776; ClinicalTrials.gov: NCT01674803.
Synergy and Promus are trademarks or registered trademarks of the Boston Scientific Group of Companies. Resolute, Resolute Onyx and Integrity are trademarks or registered trademarks of the Medtronic Group of Companies. Xience and Xience Sierra are trademarks or registered trademarks of the Abbott Group of Companies. Ultimaster is a trademark or registered trademark of the Terumo Group of Companies. BioMatrix is a trademark or registered trademark of the Biosensors International Group.
Orsiro and Orsiro Mission are trademarks or registered trademarks of the BIOTRONIK Group of Companies.
Disclaimer
Clinical data conducted with Orsiro, Orsiro Mission’s predecessor device can be used to illustrate Orsiro Mission clinical outcomes.
Orsiro Mission is currently not available in the US.
© 2021 BIOTRONIK AG – All rights reserved.
Specifications are subject to modification, revision and improvement.